

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (12): 2700.doi: 10.7503/cjcu20180481
• Physical Chemistry • Previous Articles Next Articles
YAN Xuan, XUE Bingchun*( ), LIU Erbao*(
), LIU Erbao*( )
)
Received:2018-07-05
Online:2018-12-03
Published:2018-11-05
Contact:
XUE Bingchun,LIU Erbao
E-mail:bcxue@sxnu.edu.cn;liueb@sxnu.edu.cn
Supported by:CLC Number:
TrendMD:
YAN Xuan,XUE Bingchun,LIU Erbao. Synthesis of Urea Ammonium Halide Cocrystal and Theoretical Study of Its Influencing Factors in Water System†[J]. Chem. J. Chinese Universities, 2018, 39(12): 2700.
| Method | a/nm | b/nm | c/nm | α/(°) | β/(°) | γ/(°) | Volume/nm3 |
|---|---|---|---|---|---|---|---|
| Solid phase honey-like channel method[ | 0.7923 | 1.7121 | 0.8072 | 90 | 90 | 90 | 1.0950 |
| Slow evaporation method | 0.7927 | 1.7152 | 0.8050 | 90 | 90 | 90 | 1.0945 |
Table 1 Comparison of unit cell parameters of urea ammonium chloride prepared by two methods
| Method | a/nm | b/nm | c/nm | α/(°) | β/(°) | γ/(°) | Volume/nm3 |
|---|---|---|---|---|---|---|---|
| Solid phase honey-like channel method[ | 0.7923 | 1.7121 | 0.8072 | 90 | 90 | 90 | 1.0950 |
| Slow evaporation method | 0.7927 | 1.7152 | 0.8050 | 90 | 90 | 90 | 1.0945 |
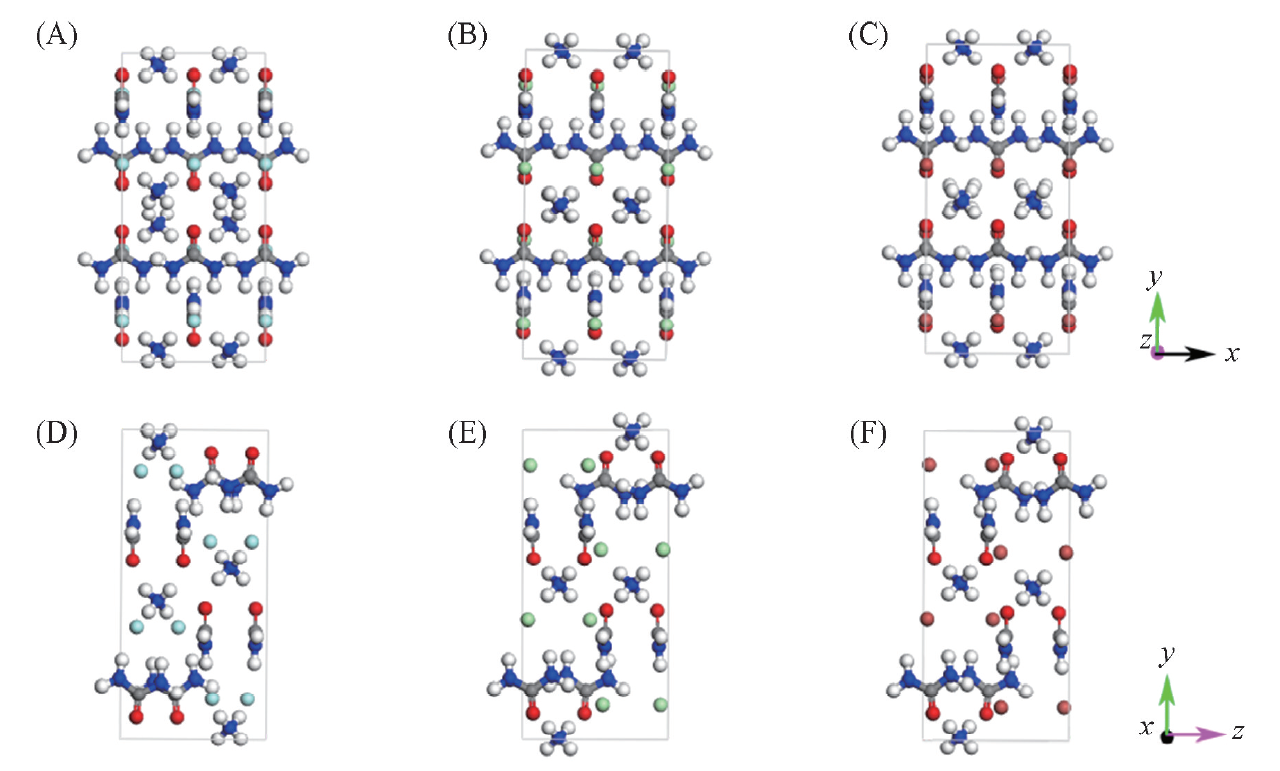
Fig.4 Comparison of three urea ammonium halide cocrystal cell structures(A, D) Urea ammonium fluoride cell structure; (B, E)urea ammonium chloride cell structure;(C, F) urea ammonium bromide cell structure.
| Species | a/nm | b/nm | c/nm | d(H1—H2)/nm | d(N1—N2)/nm | d(X1—H4)/nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea-NH4F | 0.7533 | 1.6318 | 0.8069 | 0.3433 | 0.3179 | 0.1709 | |||||
| Urea-NH4Cl | 0.8082 | 1.7301 | 0.8580 | 0.3998 | 0.3417 | 0.2253 | |||||
| Urea-NH4Br | 0.8250 | 1.7844 | 0.8875 | 0.4171 | 0.3438 | 0.2425 | |||||
| Species | d(X1—X4)/nm | d(X2—X3)/nm | d(C2—C3)/nm | d(O1—O2)/nm | ÐH3—N3—H4/(°) | ÐH4—N3—H5/(°) | |||||
| Urea-NH4F | 0.4690 | 0.4370 | 0.4343 | 0.4506 | 110.976 | 111.331 | |||||
| Urea-NH4Cl | 0.5315 | 0.5075 | 0.4937 | 0.4830 | 108.549 | 110.592 | |||||
| Urea-NH4Br | 0.5476 | 0.5220 | 0.5025 | 0.4892 | 108.162 | 110.392 | |||||
Table 2 Unit cell parameters of three units and structural parameters between halogen ions and the main atoms around them
| Species | a/nm | b/nm | c/nm | d(H1—H2)/nm | d(N1—N2)/nm | d(X1—H4)/nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea-NH4F | 0.7533 | 1.6318 | 0.8069 | 0.3433 | 0.3179 | 0.1709 | |||||
| Urea-NH4Cl | 0.8082 | 1.7301 | 0.8580 | 0.3998 | 0.3417 | 0.2253 | |||||
| Urea-NH4Br | 0.8250 | 1.7844 | 0.8875 | 0.4171 | 0.3438 | 0.2425 | |||||
| Species | d(X1—X4)/nm | d(X2—X3)/nm | d(C2—C3)/nm | d(O1—O2)/nm | ÐH3—N3—H4/(°) | ÐH4—N3—H5/(°) | |||||
| Urea-NH4F | 0.4690 | 0.4370 | 0.4343 | 0.4506 | 110.976 | 111.331 | |||||
| Urea-NH4Cl | 0.5315 | 0.5075 | 0.4937 | 0.4830 | 108.549 | 110.592 | |||||
| Urea-NH4Br | 0.5476 | 0.5220 | 0.5025 | 0.4892 | 108.162 | 110.392 | |||||
| Atom | Urea-NH4F | Urea-NH4Cl | Urea-NH4Br | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | |
| H1 | 0.0272 | 0.0661 | 0.0436 | 0.0253 | 0.0667 | 0.0437 | 0.0247 | 0.0667 | 0.0432 |
| H2 | 0.0728 | 0.0661 | 0.0436 | 0.0747 | 0.0667 | 0.0437 | 0.0753 | 0.0667 | 0.0432 |
| N1 | 0.0153 | 0.0691 | 0.0425 | 0.0143 | 0.0695 | 0.0422 | 0.0140 | 0.0694 | 0.0415 |
| N2 | 0.0347 | 0.0691 | 0.0075 | 0.0357 | 0.0695 | 0.0078 | 0.0360 | 0.0694 | 0.0086 |
| X1 | 0.0500 | 0.0632 | 0.0577 | 0.0500 | 0.0617 | 0.0549 | 0.0500 | 0.0616 | 0.0547 |
| H4 | 0.0337 | 0.0579 | 0.0678 | 0.0320 | 0.0540 | 0.0676 | 0.0315 | 0.0532 | 0.0676 |
| X2 | 0.0500 | 0.0862 | 0.0113 | 0.0500 | 0.0881 | 0.0071 | 0.0500 | 0.0883 | 0.0070 |
| X3 | 0 | 0.0862 | 0.0387 | 0 | 0.0881 | 0.0429 | 0 | 0.0883 | 0.0430 |
| C2 | 0.0500 | 0.0849 | 0.0616 | 0.0500 | 0.0843 | 0.0585 | 0.0500 | 0.0843 | 0.0588 |
| C3 | 0 | 0.0849 | 0.0884 | 0 | 0.0843 | 0.0915 | 0 | 0.0843 | 0.0912 |
| X4 | 0 | 0.0632 | 0.0923 | 0 | 0.0617 | 0.0951 | 0 | 0.0616 | 0.0953 |
| O1 | 0.0500 | 0.05670 | 0.0097 | 0.0500 | 0.0581 | 0.0096 | 0.0500 | 0.0584 | 0.0102 |
| O2 | 0 | 0.0570 | 0.0403 | 0 | 0.0581 | 0.0404 | 0 | 0.0584 | 0.0398 |
Table 3 Atomic coordinates of the main numbered atoms
| Atom | Urea-NH4F | Urea-NH4Cl | Urea-NH4Br | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | |
| H1 | 0.0272 | 0.0661 | 0.0436 | 0.0253 | 0.0667 | 0.0437 | 0.0247 | 0.0667 | 0.0432 |
| H2 | 0.0728 | 0.0661 | 0.0436 | 0.0747 | 0.0667 | 0.0437 | 0.0753 | 0.0667 | 0.0432 |
| N1 | 0.0153 | 0.0691 | 0.0425 | 0.0143 | 0.0695 | 0.0422 | 0.0140 | 0.0694 | 0.0415 |
| N2 | 0.0347 | 0.0691 | 0.0075 | 0.0357 | 0.0695 | 0.0078 | 0.0360 | 0.0694 | 0.0086 |
| X1 | 0.0500 | 0.0632 | 0.0577 | 0.0500 | 0.0617 | 0.0549 | 0.0500 | 0.0616 | 0.0547 |
| H4 | 0.0337 | 0.0579 | 0.0678 | 0.0320 | 0.0540 | 0.0676 | 0.0315 | 0.0532 | 0.0676 |
| X2 | 0.0500 | 0.0862 | 0.0113 | 0.0500 | 0.0881 | 0.0071 | 0.0500 | 0.0883 | 0.0070 |
| X3 | 0 | 0.0862 | 0.0387 | 0 | 0.0881 | 0.0429 | 0 | 0.0883 | 0.0430 |
| C2 | 0.0500 | 0.0849 | 0.0616 | 0.0500 | 0.0843 | 0.0585 | 0.0500 | 0.0843 | 0.0588 |
| C3 | 0 | 0.0849 | 0.0884 | 0 | 0.0843 | 0.0915 | 0 | 0.0843 | 0.0912 |
| X4 | 0 | 0.0632 | 0.0923 | 0 | 0.0617 | 0.0951 | 0 | 0.0616 | 0.0953 |
| O1 | 0.0500 | 0.05670 | 0.0097 | 0.0500 | 0.0581 | 0.0096 | 0.0500 | 0.0584 | 0.0102 |
| O2 | 0 | 0.0570 | 0.0403 | 0 | 0.0581 | 0.0404 | 0 | 0.0584 | 0.0398 |
| Urea cocrystal | Packing coefficient | Eform/(kJ·mol-1) | ||
|---|---|---|---|---|
| Urea | Cocrystal | NH4X | ||
| Urea-NH4F | 0.8574 | 0.9541 | 0.5487 | -6.2549 |
| Urea-NH4Cl | 0.8574 | 0.9390 | 1.0000 | -40.7346 |
| Urea-NH4Br | 0.8574 | 0.9034 | 1.0000 | -34.9912 |
Table 4 Formation energy and crystal stacking coefficient of three urea ammonium halide cocrystals
| Urea cocrystal | Packing coefficient | Eform/(kJ·mol-1) | ||
|---|---|---|---|---|
| Urea | Cocrystal | NH4X | ||
| Urea-NH4F | 0.8574 | 0.9541 | 0.5487 | -6.2549 |
| Urea-NH4Cl | 0.8574 | 0.9390 | 1.0000 | -40.7346 |
| Urea-NH4Br | 0.8574 | 0.9034 | 1.0000 | -34.9912 |
| X-(H2O)n | RX—H/nm | DE/(kJ·mol-1) |
|---|---|---|
| F-(H2O)5 | 0.1669 | -365.6760 |
| Cl-(H2O)6 | 0.2287 | -240.5609 |
| Br-(H2O)6 | 0.2456 | -249.2675 |
Table 5 Average distance of hydrogen bonds O—H…X in the first hydrated layer and binding energy(DE) of three hydrated ion clusters
| X-(H2O)n | RX—H/nm | DE/(kJ·mol-1) |
|---|---|---|
| F-(H2O)5 | 0.1669 | -365.6760 |
| Cl-(H2O)6 | 0.2287 | -240.5609 |
| Br-(H2O)6 | 0.2456 | -249.2675 |
| [1] | Lara O.F., Espinosa P.G., Supramol. Chem.,2007, 19(8), 553—557 |
| [2] | Shan N., Zaworotko M.J., Drug Discov. Today,2008, 13, 440—446 |
| [3] | Zhao G.Z., Fan R.R., Yan X.L., Yang W., Tang M.F., Jia J.F., Wu H.S., Chem. J. Chinese Universities,2018, 39(2), 292—298 |
| (赵国政, 范荣荣, 颜熹琳, 唐维, 唐明峰, 贾建峰, 武海顺. 高等学校化学学报, 2018, 39(2), 292—298) | |
| [4] | Luo Y.H., Sun B.W., Spectrochim. Acta A,2014, 120, 228—236 |
| [5] | Ghosh S., Bag P.P., Reddy C.M., Cryst. Growth Des., 2011, 11(8), 3489—3503 |
| [6] | Ross S.A., Lamprou D.A., Douroumis D., Chem. Commun.,2016, 52, 8772—8786 |
| [7] | Hebbar H.U., Rastogi N.K., Subramanian R., Int. J. Food Prop.,2008, 11(4), 804—819 |
| [8] | Rusa C.C., Tonelli A.E., Macromolecules,2000, 33, 1813—1818 |
| [9] | Tian H., Zhang M.L., Wang L.S., Tong B.H., Zhao Z., Chem. J. Chinese Universities,2018, 39(6), 1191—1196 |
| (田欢, 张梦龙, 王莉莎, 童碧海, 赵卓. 高等学校化学学报, 2018, 39(6), 1191—1196) | |
| [10] | Yang Z.W., Zhang Y.L., Li H.Z., Chinese J. Energ. Mater.,2012, 20(6), 674—679 |
| (杨宗伟, 张艳丽, 李洪珍. 含能材料, 2012, 20(6), 674—679) | |
| [11] | Thottempudi V., Shreeve J.M., J. Am.Chem. Soc.,2011, 49, 19982—19992 |
| [12] | Liu Z.Y., Xue Q.H., Bulletin Chinese Ceram. Soc.,2010, 29(5), 1041—1044 |
| (刘振英, 薛群虎. 硅酸盐通报, 2010, 29(5), 1041—1044) | |
| [13] | Maulny A. P.E., Beckett S.T., Mackenzi G., J. Food Sci.,2005, 70(9), 567—572 |
| [14] | Xue D.F., Kitamura K.J., Wang J.Y., Opt. Mater.,2003, 23, 399—402 |
| [15] | Skoepová E., Hušák M., Čejka J., Zámostný P., Kratochvíl B., J. Cryst. Growth,2014, 399, 19—26 |
| [16] | Vinothkumar P., Rajeswari K., Kumar R.M., Bhaskaran A., Spectrochim. Acta A,2015, 145(1), 33—39 |
| [17] | Yang J.Q., Li S.X., Zhao H.W., Song B., Zhang G.X., Zhang J.B., Zhu Y.M., Han J.G., J. Phys. Chem. A,2014, 118, 10927—10933 |
| [18] | Buanz A. B.M., Parkinson G.N., Gaisford S., Cryst. Growth Des., 2011, 11(4), 1177—1181 |
| [19] | Lashua A.F., Smith T.M., Hu H.G., Wei L.H., Allis D.G., Sponsler M.B., Hudson B.S., Cryst. Growth Des., 2013, 13(9), 3852—3855 |
| [20] | Rightmire N.R., Hanusa T.P., Dalton Trans.,2016, 45, 2352—2362 |
| [21] | Xue B.C., Mao M.L., Liu Y.H., Guo J.Y., Li J., Liu E.B., J. Cryst. Growth,2016, 442, 110—113 |
| [22] | Segall M., Lindan P.J., Probert M., Pickard C., Hasnip P., Clark S., Payne M., J. Phys. Condens. Mat.,2002, 14, 2717—2744 |
| [23] | Frisch M, Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision B.01, Gaussian Inc., Wallingford CT, 2010 |
| [24] | Zhang A.B., Cao Y.F., Ma Y., Zhu Y.Q., Zhang C.Y., Chinese J. Energ. Mater.,2015, 23(9), 848—857 |
| (张安帮, 曹耀峰, 马宇, 朱元强, 张朝阳. 含能材料, 2015, 23(9), 848—857 | |
| [25] | Wang J., Xia S.W., Yu L.M., Acta Chim. Sinica,2013, 71, 1307—1312 |
| (王娟, 夏树伟, 于良民. 化学学报, 2013, 71, 1307—1312 | |
| [26] | Krekeler C., Hess B., Site L.D., J. Chem. Phys.,2006, 125, 054305 |
| [1] | ZHOU Ying, HE Peinan, FENG Haisong, ZHANG Xin. Optimal Distribution of Active Sites of CO2 Reduction Reaction Catalyzed by Diatomic Site M-N-C [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210640. |
| [2] | WU Fangling,CHU Yanqiu,CHEN Xin,WEI Wanghui,DING Chuanfan. Critical Factors Affecting Noncovalent Interaction Between Pentapeptides Explored by Electrospray Ionization Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1927. |
| [3] | YAN Xiaoqing, LIU Qiushuang, LIU Yunfeng, NIU Qiao. Adsorption of Iodoperfluoroalkanes on Tetrabutyl Ammonium Halide by Halogen Bond† [J]. Chem. J. Chinese Universities, 2018, 39(4): 743. |
| [4] | YU Ao, WANG Hui-Kai, XUE Xiao-Song, CAI Yu, WANG Yong-Jian, HE Jia-Qi. Theoretical Study on the Thermodynamic Hydricity of Imidazole-based Organic Hydrides in Acetonitrile [J]. Chem. J. Chinese Universities, 2012, 33(02): 276. |
| [5] | WANG Feng, LI Wen-Hong*, LI Dong, YAN Sui-Hong, XU Kang-Zhen, SUN Xiao-Hong. Knoevenagel Reaction of 2-Aminothiophen-4-one [J]. Chem. J. Chinese Universities, 2011, 32(4): 903. |
| [6] | JI Chang-Chun, XU Zheng-Jiang, LI Jing, LIU Guang-Xiang, ZHENG He-Gen*. Syntheses, Structures of Four New Complexes Constructed by Flexible Pyridine Ligand and the Relationship Between Conformations and Structures [J]. Chem. J. Chinese Universities, 2010, 31(5): 867. |
| [7] | YU Qing, CAO Jie*, ZHANG Cheng-Gen. Direct Experimental Evidence of Ternary Diastereomeric Complexes in Chiral Separations of Phenylglycine Enatiomers with Chiral Mobile Phase by RP-HPLC [J]. Chem. J. Chinese Universities, 2009, 30(5): 988. |
| [8] | HUANG Rong-Yi, CHEN Hong, YAN Juan, ZHU Kun, LIU Guang-Xiang*, REN Xiao-Ming. Syntheses, Structures and Theoretical Calculations of Three Novel Cu(Ⅱ) Complexes [J]. Chem. J. Chinese Universities, 2009, 30(4): 655. |
| [9] | ZHANG Xin, HUANG Ting-Ting, TAN Kai, LIN Meng-Hai*, ZHANG Qian-Er. Theoretical Studies on Third-order Nonlinear Optical Property of (ZnS)6—12 Clusters [J]. Chem. J. Chinese Universities, 2007, 28(6): 1126. |
| [10] | SUN Jian-Min, WANG Lu ,WANG Ya-Li, QU Xue-Jian, JIANG Da-Zhen, XIAO Feng-Shou. Synthesis of Styrene Carbonate Catalyzed Efficiently by Zinc Bromide and Tetra-n-butylammonium Halides [J]. Chem. J. Chinese Universities, 2007, 28(3): 502. |
| [11] | MAO Hua-Ping1, YU You-Hai1, CHEN Liang1, LU Xiao-Feng1, ZHANG Wan-Jin1, ZHANG Hong-Xing2. Synthesis and Characterization of a Novel Azo Compound with ‘Dumbbell Shape’ [J]. Chem. J. Chinese Universities, 2006, 27(10): 1999. |
| [12] | HUANG Kun-Lin, LI Cai-Jin, LIU Qun, BAI Hong-Tao, MU Zhong-Cheng . Synthesis, Single Crystal Structure and 1H NMR Characterization of 3-(2-p-Methylphenylvinyl)-5(4H)-isoxazolone and Theoretical Study for Its Isomerization [J]. Chem. J. Chinese Universities, 2004, 25(7): 1264. |
| [13] | CHEN Yi-Qing, SUN Duo-Xian, SU Jing, YANG Jun . Theoretical Calculation and Experiments of Diameter of Calcium Alginate Gel Microspheres [J]. Chem. J. Chinese Universities, 2003, 24(3): 481. |
| [14] | LIU YongDong, SUN RenAn, WANG ChangSheng . Theoretical Studies of the Chemisorption of CO on Supported-metal Catalysts of Ru, Rh, Pd(Ⅱ) [J]. Chem. J. Chinese Universities, 2001, 22(12): 2094. |
| [15] | MO Yi, LI Le-Min . Influence of the Computation Conditions on the Results in Density Functional Calculations [J]. Chem. J. Chinese Universities, 2001, 22(1): 81. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||