

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (1): 47.doi: 10.7503/cjcu20180421
• Analytical Chemistry • Previous Articles Next Articles
LI Wenhong1, WANG Danyang2, CAO Jinjin2, WEI Yongju2,*( )
)
Received:2018-06-08
Online:2019-01-10
Published:2018-11-16
Contact:
WEI Yongju
E-mail:weiyju@126.com
Supported by:TrendMD:
LI Wenhong,WANG Danyang,CAO Jinjin,WEI Yongju. Comparative Study of Absorption and Fluorescence Spectra of Glycitein and Glycitin†[J]. Chem. J. Chinese Universities, 2019, 40(1): 47.
| pH | A | pKa1 | Average(pKa1) |
|---|---|---|---|
| 6.85 | 0.2824 | 7.14 | 7.08±0.04 |
| 6.94 | 0.3206 | 7.10 | 7.08±0.04 |
| 7.00 | 0.3475 | 7.08 | 7.08±0.04 |
| 7.04 | 0.3856 | 7.01 | 7.08±0.04 |
Table 1 Determination of pKa1 of glycitein*
| pH | A | pKa1 | Average(pKa1) |
|---|---|---|---|
| 6.85 | 0.2824 | 7.14 | 7.08±0.04 |
| 6.94 | 0.3206 | 7.10 | 7.08±0.04 |
| 7.00 | 0.3475 | 7.08 | 7.08±0.04 |
| 7.04 | 0.3856 | 7.01 | 7.08±0.04 |
| pH | A | pKa2 | Average(pKa2) |
|---|---|---|---|
| 9.74 | 0.5511 | 9.95 | 9.96±0.01 |
| 9.86 | 0.5423 | 9.96 | 9.96±0.01 |
| 10.02 | 0.5311 | 9.97 | 9.96±0.01 |
| 10.21 | 0.5161 | 9.96 | 9.96±0.01 |
Table 2 Determination of pKa2 of glycitein*
| pH | A | pKa2 | Average(pKa2) |
|---|---|---|---|
| 9.74 | 0.5511 | 9.95 | 9.96±0.01 |
| 9.86 | 0.5423 | 9.96 | 9.96±0.01 |
| 10.02 | 0.5311 | 9.97 | 9.96±0.01 |
| 10.21 | 0.5161 | 9.96 | 9.96±0.01 |
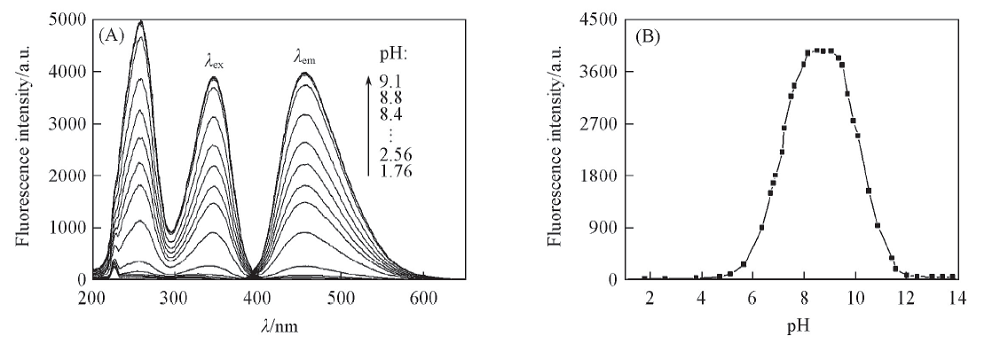
Fig.3 Fluorescence spectra of glycitein aqueous solutions at different pH values(A) and relationship between fluorescence intensity and pH value(B) c: 1.56 μg/mL, volume fraction of MeOH: 10%, λex/λem: 347 nm/456 nm.
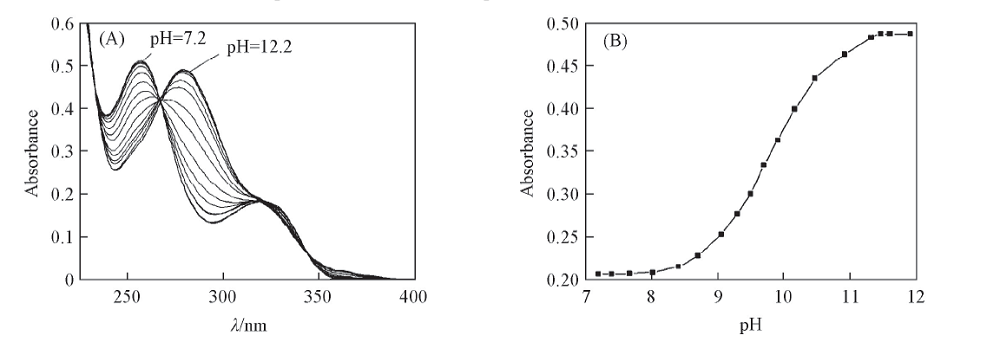
Fig.4 Absorption spectra of glycitin aqueous solutions at different pH values(A) and relationship between absorbance and pH value(B) c: 8.20 μg/mL, volume fraction of MeOH: 10%, λ=280 nm.
| pH | A | pKa | Average(pKa) |
|---|---|---|---|
| 9.63 | 0.3114 | 9.85 | 9.81±0.03 |
| 9.79 | 0.3342 | 9.87 | 9.81±0.03 |
| 9.90 | 0.3633 | 9.80 | 9.81±0.03 |
| 10.12 | 0.3946 | 9.81 | 9.81±0.03 |
Table 3 Determination of pKa of glycitin*
| pH | A | pKa | Average(pKa) |
|---|---|---|---|
| 9.63 | 0.3114 | 9.85 | 9.81±0.03 |
| 9.79 | 0.3342 | 9.87 | 9.81±0.03 |
| 9.90 | 0.3633 | 9.80 | 9.81±0.03 |
| 10.12 | 0.3946 | 9.81 | 9.81±0.03 |
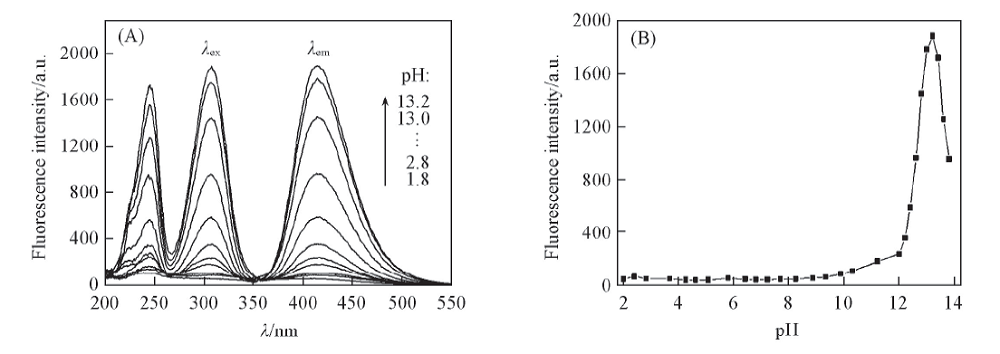
Fig.5 Fluorescence spectra of glycitin alkaline solutions at different pH values(A) and influence of pH on fluorescence intensity(B) c: 1.68 μg/mL, volume fraction of MeOH: 10%, heat for 0.5 h at 100 ℃, λex/λem: 307 nm/415 nm.
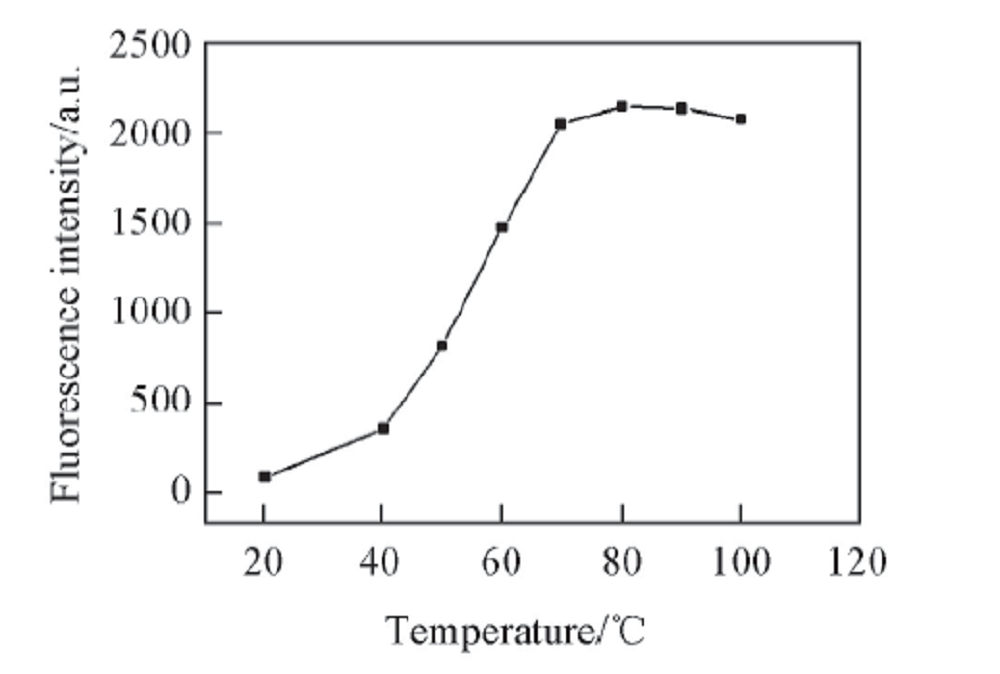
Fig.7 Relationship between fluorescence intensity and heating temperaturec: 1.68 μg/mL, volume fraction of MeOH: 10%, heat for 0.5 h, pH=13.2, λex/λem: 307 nm/415 nm.
| [1] | Singleton J. A., Stikeleather L. F., Sanford J. H., J. Am. Oil Chem. Soc.,2002, 79(8), 741—748 |
| [2] | Kim E. Y., Hong K. B., Suh H. J., Choi H. S., Food Funct.,2015, 6(11), 3512—3521 |
| [3] | Shi S. Y., Ma Y. J., Zhang Y. P., Liu L. L., Liu Q., Peng M. J., Xiong X., Sep. Purif. Technol.,2012, 89(2), 225—233 |
| [4] | Wei Q. K., Jone W. W., Fang T. J., J. Food and Drug Anal.,2004, 12(4), 324—331 |
| [5] | Ma X. M., Guo S. R., Duan Z. W., Wang X. Y., Li X., Chinese Traditional and Herbal Drugs,2007, 38(11), 1645—1647 |
| (马学敏, 郭树仁, 段震文, 王祥云, 李霄. 中草药, 2007, 38(11), 1645—1647) | |
| [6] | Nagata Y., Sugiyama Y., Fukuta F., Takayanagi A., Masumori N., Tsukamoto T., Akasaka H., Ohnishi H., Saitoh S., Miura T., Moriyama K., Tsuji H., Akaza H., Mori M., Int. Urol. Nephrol.,2016, 48, 1453—1460 |
| [7] | Pyo Y. H., J. Food Science and Nutrition, 2007, 12, 26—34 |
| [8] | Pyo Y. H., Seong K. S., J. Agricultural and Food Chemistry,2009, 57, 8617—8622 |
| [9] | Li Y., Zhang H., Food Funct.,2017, 8, 2935—2944 |
| [10] | Glabska D., Guzek D., Grudzinska D., Lech G., World J. Gastroenterol,2017, 23(29), 5356—5363 |
| [11] | Kwon D. Y., Daily J. W. I., Kim H. J., Park S., Nutrition Research,2010, 30, 1—13 |
| [12] | Puri A., Panda B.P.,J. Chromatographic Science, 2014, (5), 1—7 |
| [13] | da Costa C. I., Braga F. C., Soares C. D. V., de Aguiar N. E., Pianetti G. A., Condessa F. A., Barbosa T. A. F., Campos L. M. M., J. Chromatogr. B,2006, 836, 74—78 |
| [14] | Qu L. P., Fan G. R., Peng J. Y., Mi H. M., Fitoterapia,2007, 78, 200—204 |
| [15] | Auwerter L. C. C., Wanczinski A. E., Chiandotti R. S., Revista Brasileira de Farmacognosia Brazilian Journal of Pharmacognosy,2012, 22(6), 1344—1348 |
| [16] | Thomas B. F., Zeisel S. H., Busby M. G., Hill J. M., Mitchell R. A., Scheffler N. M., Brown S. S., Bloeden L. T., Dix K. J., Robert Jeffcoat A., J. Chromatogr. B,2001, 760(2), 191—205 |
| [17] | Wei Y. J., Li N., Qin S. J., Spectroscopy and Spectral Analysis,2004, 24(6), 647—651 |
| (魏永巨, 李娜, 秦身钧. 光谱学与光谱分析, 2004, 24(6), 647—651) | |
| [18] | Li L. R., Liu C. G., Wei Y. J., Spectroscopy and Spectral Analysis,2011, 31(10), 2763—2766 |
| (李丽然, 刘翠格, 魏永巨. 光谱学与光谱分析, 2011, 31(10), 2763—2766) | |
| [19] | Li W. H., Sun C. M., Wei Y. J.,Acta Pharmaceutica Sinica,2015, 50(10), 1324—1329 |
| (李文红, 孙冲梅, 魏永巨. 药学学报, 2015, 50(10), 1324—1329) | |
| [20] | Li W. H., Cao J. J., Lu R., Wei Y. J., Spectroscopy and Spectral Analysis,2016, 36(4), 1007—1012 |
| (李文红, 曹津津, 卢蕊, 车翠霞, 魏永巨. 光谱学与光谱分析, 2016, 36(4), 1007—1012) | |
| [21] | Zhang P.C., Flavonoids Chemistry, Chemical Industry Press, Beijing, 2009, 231—232 |
| (张培成. 黄酮化学, 北京:化学工业版社, 2009, 231—232) | |
| [22] | Pei X., Zhao J., Cai P., Sun W., Ren J., Wu Q., Zhang S., Tian C., Protein Expression and Purification,2016, 119, 75—84 |
| [23] | Prasad L. N., Shah N., International Food Research J.,2011, 19(2), 433—439 |
| [1] | LI Ting, CAO Zhong, LI Panpan, HE Jinglin, XIAO Hui, YANG Chan. High-sensitive Fluorescent Enhancement Detection of Hg(Ⅱ) Ions Based on Poly(thymine)-templated Copper Nanoclusters† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1616. |
| [2] | DAI Yulin, YU Shanshan, ZHANG Ying, HAO Ying, ZHONG Wei, YUE Hao, LIU Shuying. Studies on the Isoflavone in Extract of the Flower of Pueraria Lobata by RRLC-Q-TOF MS/MS† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1396. |
| [3] | HAN Bing-Xing, WU Fang-Ying. Synthesis of N,N-Dimethyl Pyridine Benzaldehyde-4-dimethylaminobenzoylhydrazone and Its Application for Selective Recognizing Cu2+ [J]. Chem. J. Chinese Universities, 2013, 34(11): 2483. |
| [4] | XIAO Min, ZHANG Li-Na, WU Fang-Ying. Synthesis of 2'-Borono-benzaldehyde-7-(8-hydroxy-5-sulfoacid) Quinoline Hydrazone and Recognition of Pb2+ [J]. Chem. J. Chinese Universities, 2012, 33(05): 919. |
| [5] | HUANG Hui, XIANG Dong-Shan, LI Li, LI Hai-Gang, ZENG Guo-Ping, HE Zhi-Ke*. Rapid and Sensitive Fluorescence Enhancement Method by Barbituric Acid Derivatives for the Determination of Melamine in Milk [J]. Chem. J. Chinese Universities, 2011, 32(11): 2504. |
| [6] | CHEN Zhan-Guo*, ZHAO Hai-Xia, WEI Jun-Fa, LIU Bo. Total Synthesis of 2-Methyl-7-[ω-(1H-imidazol-1-yl)ethoxy]-Isoflavone Derivatives and Their Antioxidative Activity [J]. Chem. J. Chinese Universities, 2009, 30(1): 82. |
| [7] | FU Guo-Liang1, FENG Feng1,2*, CHEN Ze-Zhong2, BAI Yun-Feng2, MENG Shuang-Ming2, LIN Sen1, JIANG Run-Sheng1. Synthesis and Analytical Application of a New Heterocyclic Triazene Fluorescent Reagent 1,8-Bis(2-benthiazolydiazoamino)naphthalene [J]. Chem. J. Chinese Universities, 2008, 29(8): 1560. |
| [8] | LI Hui1, WAN Le-Ren2, WANG Hong1, Hashi Yuki2*, CHEN Shi-Zhong1*. Identification and Mass Spectrometric Characterization of Isomeric Isoflavone Aglycones by ESI-IT-TOF Mass Spectrometry [J]. Chem. J. Chinese Universities, 2007, 28(12): 2284. |
| [9] | YANG Man-Man, XI Xiao-Li, YANG Pin. Studies on Interaction of New Medicine of QuinoloneClass with HSA and BSA by Using Fluorescence Enhancement and Fluorescence Quenching Theory [J]. Chem. J. Chinese Universities, 2006, 27(4): 687. |
| [10] | ZHANG Zun-Ting, WANG Qiu-Ya, HE Yun, WANG Xiao-Bing, XUE Dong, ZHENG Jian-Bin. Syntheses,Crystal Structures and Biological Activity of Bimethylation Daidzein Sulfonates [J]. Chem. J. Chinese Universities, 2005, 26(12): 2247. |
| [11] | ZHANG Zun-Ting, WANG Qiu-Ya, HE Yun, WANG Xiao-Bing, XUE Dong, ZHENG Jian-Bin. Syntheses,Crystal Structures and Biological Activity of Bimethylation Daidzein Sulfonates [J]. Chem. J. Chinese Universities, 2005, 26(12): 2247. |
| [12] | LIU Qian-Guang, ZHANG Zun-Ting, XUE Dong. Synthesis, Crystal Structure and Activity of Sul fated Daidzein [J]. Chem. J. Chinese Universities, 2003, 24(5): 820. |
| [13] | SUN Zhao-Yong, AI Xi-Cheng, FENG Juan, ZHANG Qi-Yuan, ZHANG Xing-Kang . Studies on the Luminescence Properties and Decay Dynamics of Eu(Ⅲ) Enhanced Fluorescence Systems in Micelle Solutions [J]. Chem. J. Chinese Universities, 2001, 22(11): 1856. |
| [14] | LIU Peng, CHEN Rong-Feng, CHANG Jun-Biao, XIE Jing-Xi . 1H NMR Studies on Synthetic Isoflavones with p-Substituents on B Ring [J]. Chem. J. Chinese Universities, 2000, 21(11): 1671. |
| [15] | HUANG Ying-Ping, CAI RU Xiu, HUANG Hou-Ping . Studies on the Fluorescence Enhancement of Reverse Micelle on 2,3-Diaminophenazine [J]. Chem. J. Chinese Universities, 1999, 20(7): 1031. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||