

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (2): 206.doi: 10.7503/cjcu20160624
• Organic Chemistry • Previous Articles Next Articles
ZHU Longbao1, TAO Yugui1, GE Fei1, LI Wanzhen1, LIU Yi2,*( ), DU Guocheng3
), DU Guocheng3
Received:2016-09-02
Online:2017-02-10
Published:2016-12-19
Contact:
LIU Yi
E-mail:myputer@163.com
Supported by:CLC Number:
TrendMD:
ZHU Longbao, TAO Yugui, GE Fei, LI Wanzhen, LIU Yi, DU Guocheng. Production and Characterization of Phenylalanine Aminomutase from Streptomyces Maritimus and Synthesis of β-Arylalanine†[J]. Chem. J. Chinese Universities, 2017, 38(2): 206.
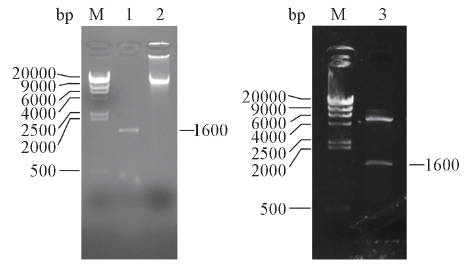
Fig.1 Agarose gel electrophoresis of gene DNA, PCR products and the digested pET28a-pamM: marker; lane 1: product of PCR; lane 2: genomeDNA; lane 3: the pET28a-pam was digested by EcoRⅠ and NdeⅠ.
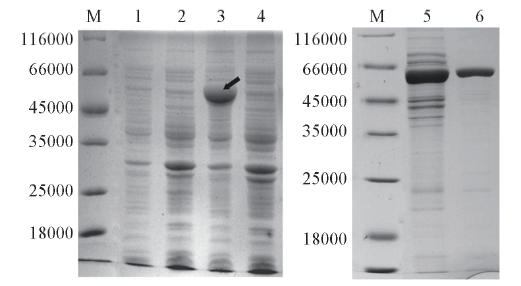
Fig.2 SDS-PAGE of recombinant enzyme expressed in E.coli BL21(DE3) and purification of the recombinant SmPAM using HisTrapTM/FFM: marker; lane 1: E.coli BL21; lane 2: cell extracts of E.coli BL21/pET28a-pam without induction by IPTG; lanes 3 and 5: the supernatant fraction after sonication of E.coli BL21/pET28a-pam induced by IPTG(the arrow in lane 3 represents the expressed SmPAM); lane 4: the precipitated fraction after sonication of E.coli BL21/pET28a-pam induced by IPTG; lane 6: the purified SmPAM by HisTrap TM/Faffinity chromatography column.
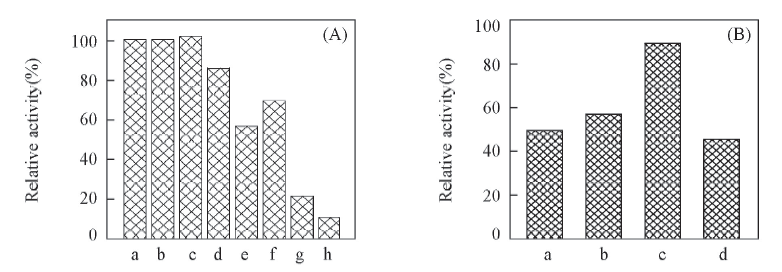
Fig.6 Effects of metal ions(A) and surfactants(B) on the activity of recombinant SmPAM(A) a. Na+; b. Mg2+; c. Ca2+; d. Fe2+; e. Cu2+; f. Zn2+; g. Mn2+; h. Co3+; (B) a. CTAB; b. Triton X-100; c. Tween 80; d. SDS.
| [1] | Kudo F., Miyanaga A ., Eguchi T., Nat. Prod. Rep., 2014, 31(8),1056—1073 |
| [2] | Grayson J. I., Roos J., Osswald S., Org. Process. Res. Dev., 2011, 15(5), 1201—1206 |
| [3] | Zhang X. H., Ye W. J., Wang K. L., TianY. S., Xiao X., Chem. Res. Chinese Universities,2015, 31(2), 203—207 |
| [4] | Ratnayake N. D., Theisen C., Walter T., Walker K. D., J. Biotechnol., 2016, 10(217), 12—21 |
| [5] | Liu J. Z., Xiong J. B., Zhao G. H., Liu Q., Jiao Q. C., Chem. J. Chinese Universities,2010, 31(11), 2234—2238 |
| (刘均忠, 熊吉滨, 赵根海, 刘茜, 焦庆才. 高等学校化学学报, 2010, 31(11), 2234—2238) | |
| [6] | Li D. C., Jia L., Wang X. F., Wei D. Z., Prep. Biochem. Biotechnol,2013, 43(2), 207—216 |
| [7] | Mathew S., Bea H., Nadarajan S. P., Chung T., Yun H., J. Biotechnol., 2015, 20(196—197), 1—8 |
| [8] | Mathew S., Jeong S. S., Chung T., Lee S. H., Yun H., Biotechnol. J., 2016, 11(1),185—190 |
| [9] | Zhang K., Liang X., He M., Wu J., Zhang Y., Xue W., Jin L., Yang S., Hu D., Molecules,2013, 18(6), 6142—6152 |
| [10] | Heberling M. M., Wu B., Bartsch S., Janssen D. B., Curr. Opin. Chem. Biol., 2013, 17(2), 250—260 |
| [11] | Lohman J. R., Shen B., Methods Enzymol., 2012, 516, 299—319 |
| [12] | Cooke H. A., Christianson C. V., Bruner S. D., Curr. Opin. Chem. Biol., 2009, 13(4), 460—468 |
| [13] | Walter T., King Z., Walker K. D.,Biochemistry,2016, 55(1), 1—4 |
| [14] | Weise N. J., Parmeggiani F., Ahmed S. T., Turner N. J., J. Am. Chem. Soc., 2015, 137(40), 12977—12983 |
| [15] | Lovelock S. L., Lloyd R. C., Turner N. J., Angew. Chem. Int. Ed. Engl., 2014, 53, 4652—4656 |
| [16] | Heberling M. M., Masman M. F., Bartsch S., Wybenga G. G., Dijkstra B. W., Marrink S. J., Janssen D. B., ACS Chem. Biol., 2015, 10(4), 989—997 |
| [17] | Zhu L. B., Cui W., Fang Y. Q., Liu Y., Gao X. X., Zhou Z. M., Biotechnol. Lett., 2013, 35, 751—756 |
| [18] | Parmeggiani F., Lovelock S. L., Weise N. J., Ahmed S. T., Turner N. J., Angew. Chem. Int. Ed. Engl., 2015, 54(15), 4608—4611 |
| [19] | Wu B., Szymanski W., de Wildeman S., Poelarends G. J., Feringa B. L., Janssen D. B., Adv. Synth. Catal., 2010, 352(9), 1409—1412 |
| [20] | Wu B., Szymanski W., Wybenga G. G., Heberling M. M., Stefaan de Wildeman S. B., Poelarends G. J., Dijkstra B. L., Bauke W., Janssen D. B., Angew. Chem. Int. Ed. Engl., 2012, 51(2), 482—486 |
| [21] | Feng L., Wanninayake U., Strom S., Geiger J., Walker K. D., Biochemistry,2011, 50(14), 2919—2930 |
| [22] | Wu B., Szymanski W., Wietzes P., de Wildeman S., Poelarends G. J., Feringa B. L., Janssen D. B., ChemBioChem,2009, 10(2), 338—344 |
| [1] | TANG Yujing, HU Min, WANG Xia, WANG Qigang. Advances in Enzyme-load Nanocatalytic Systems for Disease Treatment [J]. Chem. J. Chinese Universities, 0, (): 20220640. |
| [2] | CHANG Liying, LING Xinyu, CHEN Heqi, WANG Xue, LIU Tao. Application of Gene Editing in Mitochondrial Diseases [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220363. |
| [3] | CAO Shujie, LI Hongjun, GUAN Wenli, REN Mengtian, ZHOU Chuanzheng. Progress on the Stereocontrolled Synthesis of Phosphorothioate Oligonucleotides [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220304. |
| [4] | XU Yongbin, FENG Shuaixia, CHEN Jie, GONG Hua, SHI Songshan, WANG Huijun, WANG Shunchun. Structural Characterization of a Homogeneous Polysaccharide Isolated From the Flower of Carthamus tinctorius L. and Its Inhibitory Activity on HepG2 Proliferation [J]. Chem. J. Chinese Universities, 0, (): 20220600. |
| [5] | WU Yushuai, SHANG Yingxu, JIANG Qiao, DING Baoquan. Research Progress of Controllable Self-assembled DNA Origami Structure as Drug Carrier [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220179. |
| [6] | LIU Wenting, LIU Liuyi, ZHU Bochen, MAO Zongwan. Progress on the Recognition, Complex Structure and Intracellular Detection of Nucleic Acid G-quadruplex [J]. Chem. J. Chinese Universities, 0, (): 20220419. |
| [7] | HU Yucan, CAO Zhaohui, ZHENG Linggang, SHEN Juntao, ZHAO Wei, DAI Lei. Application of CRISPR-Cas Technologies in Microbiome Engineering [J]. Chem. J. Chinese Universities, 0, (): 20220362. |
| [8] | FANG Xin, ZHAO Ruiqi, MO Jing, WANG Yafen, WENG Xiaocheng. Sequencing Methods for Detection of Nucleic Acid Epigenetic Modifications [J]. Chem. J. Chinese Universities, 0, (): 20220342. |
| [9] | ZHANG Kaisong, WANG Shaoru, ZHANG Yutong, TIAN Tian. Study of Epigenetic Modifications of Nucleic Acids Based on Supramolecular Chemistry [J]. Chem. J. Chinese Universities, 0, (): 20220335. |
| [10] | ZHU Kai, LI Jie, WU Xiaoyi, HU Weiwei, WU Dongmei, YU Chengxiao, GE Zhiwei, YE Xingqian, CHEN Shiguo. Combined PGC-Triple-Tof-MS Enables the Separation, Identification of Sugar Beet Pectin Derived Oligomers [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220023. |
| [11] | FU Jun, WU Meichan, WANG Shuzhen, SHAO Xiuli, HE Feng. Antifungal Mechanism of Fubaiju Essential Oil According to Labeling Method [J]. Chem. J. Chinese Universities, 2021, 42(12): 3657. |
| [12] | ZHAO Zhuo, WANG Xueqiang. Investigations upon the Bioconjugation-based Construction Technologies and Applications of Aptamer-drug Conjugates [J]. Chem. J. Chinese Universities, 2021, 42(11): 3367. |
| [13] | CHEN Wang, HU Daihua, LIU Gege. Synthesis of Ursodeoxycholic Acid from Dehydroiso-androsterone 3-Acetate [J]. Chem. J. Chinese Universities, 2021, 42(9): 2782. |
| [14] | HU Haocheng, LI Wenli, ZHANG Jianing, LIU Yubo. Extraction, Structure Characterization and Biological Activities of Oligosaccharides from Auricularia heimuer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2465. |
| [15] | YANG Yiran, YAO Hua, YAN Jianghong, SUN Zhiheng, ZHANG Yu, FANG Xueqing, LI Xuwen, JIN Yon⁃Ri. Chemical Constituents of New Steroidal Saponins from Allium chinense G. Don [J]. Chem. J. Chinese Universities, 2021, 42(6): 1742. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||