

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1542.doi: 10.7503/cjcu20160326
• Polymer Chemistry • Previous Articles Next Articles
RONG Jiameng, ZHOU Cao, XU Dong, SUN Wei, HUANG Xiaowen, ZHANG Xingyuan*( )
)
Received:2016-05-10
Online:2016-07-14
Published:2016-07-14
Contact:
ZHANG Xingyuan
E-mail:zxym@ustc.edu.cn
CLC Number:
TrendMD:
RONG Jiameng,ZHOU Cao,XU Dong,SUN Wei,HUANG Xiaowen,ZHANG Xingyuan. Dual Light-emitting Properties of Hydroxyl-terminated Poly(lactic acid) Based on Benzophenone Derivatives†[J]. Chem. J. Chinese Universities, 2016, 37(8): 1542.
| Sample | mNBP(NBP-Cl)/g | mD,L-Lactide/g | Sample | mNBP(NBP-Cl)/g | mD,L-Lactide/g | ||
|---|---|---|---|---|---|---|---|
| NBP-PLA1 | 0.15 | 14.85 | 0.30 | NBP-Cl-PLA1 | 0.15 | 14.85 | 0.30 |
| NBP-PLA2 | 0.30 | 14.50 | 0.35 | NBP-Cl-PLA2 | 0.30 | 14.50 | 0.35 |
| NBP-PLA5 | 0.45 | 8.55 | 0.18 | NBP-Cl-PLA5 | 0.45 | 8.55 | 0.18 |
| NBP-PLA10 | 1.20 | 10.50 | 0.23 | NBP-Cl-PLA10 | 1.20 | 10.50 | 0.23 |
Table 1 Synthesis of NBP-PLA with different contents of NBP and NBP-Cl-PLA with different contents of NBP-Cl
| Sample | mNBP(NBP-Cl)/g | mD,L-Lactide/g | Sample | mNBP(NBP-Cl)/g | mD,L-Lactide/g | ||
|---|---|---|---|---|---|---|---|
| NBP-PLA1 | 0.15 | 14.85 | 0.30 | NBP-Cl-PLA1 | 0.15 | 14.85 | 0.30 |
| NBP-PLA2 | 0.30 | 14.50 | 0.35 | NBP-Cl-PLA2 | 0.30 | 14.50 | 0.35 |
| NBP-PLA5 | 0.45 | 8.55 | 0.18 | NBP-Cl-PLA5 | 0.45 | 8.55 | 0.18 |
| NBP-PLA10 | 1.20 | 10.50 | 0.23 | NBP-Cl-PLA10 | 1.20 | 10.50 | 0.23 |
| Sample | Degree of polymerization | Molecular weight(actual value of NMR) | PDI | ||||
|---|---|---|---|---|---|---|---|
| Theoretical | Actual | Theoretical | Actual | ||||
| NBP-PLA1 | 350.6 | 330.6 | 25500 | 24000 | 34200 | 58000 | 1.69 |
| NBP-PLA2 | 171.2 | 155 | 12500 | 11400 | 18100 | 23000 | 1.27 |
| NBP-PLA5 | 67.3 | 76.7 | 5100 | 5700 | 9400 | 12000 | 1.27 |
| NBP-PLA10 | 30.9 | 37.2 | 2400 | 2900 | 5200 | 6600 | 1.26 |
| NBP-Cl-PLA1 | 397.4 | 356.7 | 28900 | 25900 | 75000 | 91000 | 1.21 |
| NBP-Cl-PLA2 | 194.0 | 195.0 | 14200 | 14300 | 29000 | 50000 | 1.72 |
| NBP-Cl-PLA5 | 76.3 | 88.0 | 5700 | 6600 | 12000 | 19000 | 1.58 |
| NBP-Cl-PLA10 | 35.1 | 36.0 | 2800 | 2800 | 8600 | 11100 | 1.39 |
Table 2 Polymerization, relative molecular mass and PDI of NBP-PLA 1—10 and NBP-Cl-PLA 1—10
| Sample | Degree of polymerization | Molecular weight(actual value of NMR) | PDI | ||||
|---|---|---|---|---|---|---|---|
| Theoretical | Actual | Theoretical | Actual | ||||
| NBP-PLA1 | 350.6 | 330.6 | 25500 | 24000 | 34200 | 58000 | 1.69 |
| NBP-PLA2 | 171.2 | 155 | 12500 | 11400 | 18100 | 23000 | 1.27 |
| NBP-PLA5 | 67.3 | 76.7 | 5100 | 5700 | 9400 | 12000 | 1.27 |
| NBP-PLA10 | 30.9 | 37.2 | 2400 | 2900 | 5200 | 6600 | 1.26 |
| NBP-Cl-PLA1 | 397.4 | 356.7 | 28900 | 25900 | 75000 | 91000 | 1.21 |
| NBP-Cl-PLA2 | 194.0 | 195.0 | 14200 | 14300 | 29000 | 50000 | 1.72 |
| NBP-Cl-PLA5 | 76.3 | 88.0 | 5700 | 6600 | 12000 | 19000 | 1.58 |
| NBP-Cl-PLA10 | 35.1 | 36.0 | 2800 | 2800 | 8600 | 11100 | 1.39 |
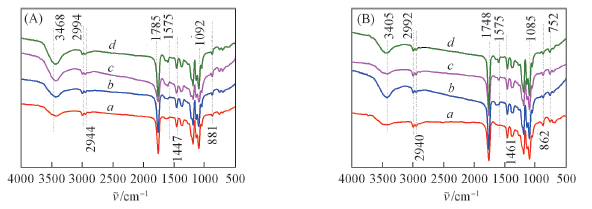
Fig.5 FTIR spectra of NBP-PLA(A) and NBP-Cl-PLA(B) (A) a. NBP-PLA1; b. NBP-PLA2; c. NBP-PLA5; d. NBP-PLA10. (B) a. NBP-Cl-PLA1; b. NBP-Cl-PLA2; c. NBP-Cl-PLA5; d. NBP-Cl-PLA10.
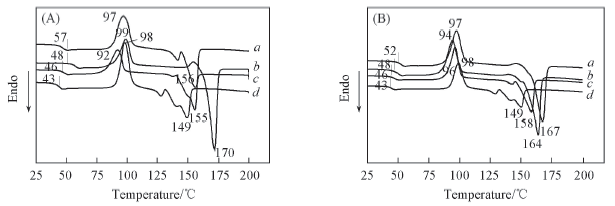
Fig.6 DSC curves of NBP-PLA1(a), NBP-PLA2(b), NBP-PLA5(c) and NBP-PLA10(d)(A) and NBP-Cl-PLA1(a), NBP-Cl-PLA2(b), NBP-Cl-PLA5(c) and NBP-Cl-PLA10(d)(B) powders under nitrogen condition
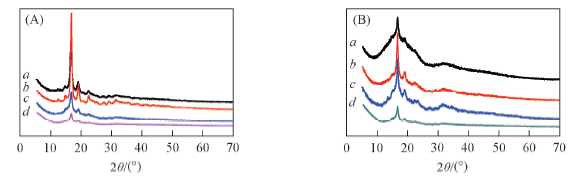
Fig.7 XRD curves of NBP-PLA1(a), NBP-PLA2(b), NBP-PLA5(c) and NBP-PLA10(d)(A) and NBP-Cl-PLA1(a), NBP-Cl-PLA2(b), NBP-Cl-PLA5(c) and NBP-Cl-PLA10(d)(B) powders
| Sample | Xc (%) | Sample | Xc(%) |
|---|---|---|---|
| NBP-PLA1 | 33.28 | NBP-Cl-PLA1 | 51.28 |
| NBP-PLA2 | 48.80 | NBP-Cl-PLA2 | 54.83 |
| NBP-PLA5 | 37.23 | NBP-Cl-PLA5 | 35.78 |
| NBP-PLA10 | 28.62 | NBP-Cl-PLA10 | 23.68 |
Table 3 Crystallinity of NBP-PLA and NBP-Cl-PLA powders*
| Sample | Xc (%) | Sample | Xc(%) |
|---|---|---|---|
| NBP-PLA1 | 33.28 | NBP-Cl-PLA1 | 51.28 |
| NBP-PLA2 | 48.80 | NBP-Cl-PLA2 | 54.83 |
| NBP-PLA5 | 37.23 | NBP-Cl-PLA5 | 35.78 |
| NBP-PLA10 | 28.62 | NBP-Cl-PLA10 | 23.68 |
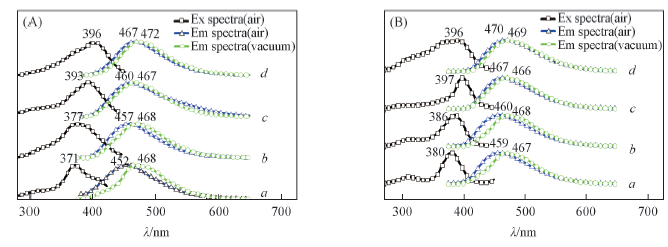
Fig.9 Photoluminescence excitation and fluorescence emission and photoluminescence emission mixed fluorescence and strong phosphoresce of NBP-PLA1(a), NBP-PLA2(b), NBP-PLA5(c) and NBP-PLA10(d)(A) and NBP-Cl-PLA1(a), NBP-Cl-PLA2(b), NBP-Cl-PLA5(c) and NBP-Cl-PLA10(d)(B) films in vacuum
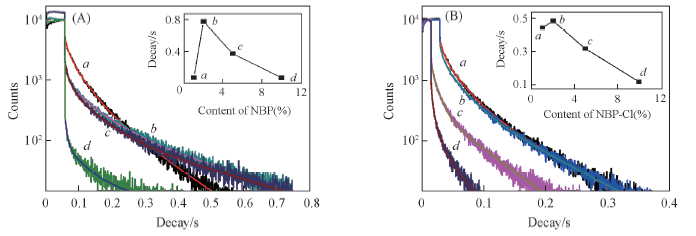
Fig.10 Phosphorescence lifetime spectra of NBP-PLA1(a), NBP-PLA2(b), NBP-PLA5(c) and NBP-PLA10(d) films(A) an NBP-Cl-PLA1(a), NBP-Cl-PLA2(b), NBP-Cl-PLA5(c) and NBP-Cl-PLA10(d) films(B) Insets: Decay vs. content of NBP.
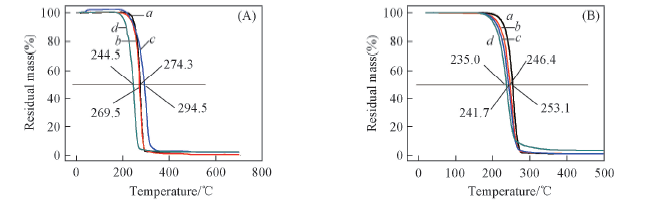
Fig.11 TGA curves of NBP-PLA1(a), NBP-PLA2(b), NBP-PLA5(c) and NBP-PLA10(d)(A) and NBP-Cl-PLA1(a), NBP-Cl-PLA2(b), NBP-Cl-PLA5(c) and NBP-Cl-PLA10(d)(B) powders
| [1] | Feng, J. , Xiong, L. , Wang, S. , Li, S. , Li, Y. , Yang, G. , Adv. Funct. Mater., 2013, 23( 3), 340- 345 |
| [2] |
Zhao, Q. , Cao, T. , Li, F. , Li, X. , Jing, H. , Yi, T. , Huang, C. , Organometallics, 2007, 26( 8), 2077- 2081
doi: 10.1021/om061031r URL |
| [3] |
Yang, Y. , Zhao, Q. , Feng, W. , Li, F. , Chemical Reviews, 2013, 113( 1), 192- 270
doi: 10.1021/cr2004103 |
| [4] | Ma, Y. , Liu, S. , Yang, H. , Wu, Y. , Yang, C. , Liu, X. , Huang W., J. , Mater. Chem., 2011, 21( 47), 18974- 18982 |
| [5] |
Xu, X. , Ye, S. , He, B. , Chen, B. , Xiang, J. , Zhou, J. , Qiu, H. , Dyes and Pigments, 2014, 101, 136- 41
doi: 10.1016/j.dyepig.2013.09.042 URL |
| [6] |
Su S., J. , Chiba, T. , Takeda, T. , Kido J., J. , Adv. Mater., 2008, 20( 11), 2125- 2130
doi: 10.1002/adma.200701730 URL |
| [7] |
Cui L., S. , Kim J., U. , Nomura, H. , Nakanotani, H. , Adachi, C. , Angew. Chem. Int. Ed., 2016, 55( 24), 6864- 6868
doi: 10.1007/s13277-016-5032-z URL pmid: 27029387 |
| [8] | Sá, nchez-Barragán I. , Costa-Ferná, ndez J. M. , Sanz-Medel, A. , Valledor, M. , Campo J., C. , TrAC-Trend. Anal. Chem., 2006, 25( 10), 958- 967 |
| [9] | Ji S., M. , Guo H., M. , Yuan X., L. , Li X., H. , Ding H., D. , Gao, P. , Zhao C., X. , Wu W., T. , Wu W., H. , Zhao J., Z. , Org. Lett., 2010, 12( 12), 2876- 2879 |
| [10] |
Kishimura, A. , Yamashita, T. , Aida, T. , J. Am. Chem. Soc., 2005, 127( 1), 179- 183
doi: 10.1021/ja0441007 URL pmid: 15631467 |
| [11] |
Lehner, P. , Staudinger, C. , Borisov S., M. , Klimant, I. , Nat., Commun. , 2014, 5 , 4460
doi: 10.1038/ncomms5460 URL pmid: 25042041 |
| [12] |
Hamai, S. , J. Chem. Soc. , Chem. Commun. , 1994, (19), 2243- 2244
doi: 10.1023/A:1007986212814 URL |
| [13] | Brewster R., E. , Kidd M. J., Schuh M. D., Chem. Commun. , 2001, (12), 1134- 1135 |
| [14] | 谢剑炜, 许金钩, 陈国珍, 刘长松. 高等学校化学学报, 1996, 17( 1), 46- 48 |
| Xie J., W. , Xu J., G. , Chen G., Z. , Liu C., S. , Chem. J. Chinese Universities, 1996, 17( 1), 46- 48 | |
| [15] |
Zhang, G. , Chen, J. , Payne S., J. , Kooi S., E. , Demas J., N. , Fraser C., L. , J. Am. Chem. Soc., 2007, 129( 29), 8942- 8943
doi: 10.1021/ja0720255 URL pmid: 17608480 |
| [16] |
Zhang, G. , Palmer G., M. , Dewhirst M., W. , Fraser C., L. , Nat. Mater., 2009, 8( 9), 747- 751
doi: 10.1038/nmat2509 URL pmid: 2846459 |
| [17] |
Zhang, X. , Xie, T. , Cui, M. , Yang, L. , Sun, X. , Jiang, J. , Zhang, G. , ACS Appl. Mater. Interfaces, 2014, 6( 4), 2279- 2284
doi: 10.1021/am405209w URL pmid: 24484404 |
| [18] |
Kong, Y. , Hay J., N. , Eur. Polym. J., 2003, 39( 8), 1721- 1727
doi: 10.1016/S0014-3057(03)00054-5 URL |
| [19] |
Wang, H. , Sun, X. , Seib P., J. , Appl. Polym. Sci., 2001, 82( 7), 1761- 1767
doi: 10.1002/app.2018 URL |
| [20] |
Lakowicz J. R., Principles of Fluorescence Spectroscopy, Plenum Press, New York, 1983, 303— 312
doi: 10.1007/978-1-4615-7658-7_3 URL |
| [21] | Zhang, G. , Fiore G., A. , Clair T. L., S. , Fraser C., L. , Macromolecules, 2009, 42( 8), 3162- 3169 |
| [1] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [2] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [3] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [4] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [5] | WU Zexin, ZHU Yuanjie, WANG Hongzhong, WANG Junan, HE Ying. Methyl-modified Carbazole/Diphenyl Sulfone-based AIE-TADF Blue Emitter and Its OLEDs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220371. |
| [6] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [7] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [8] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [9] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [10] | LI Ran, ZHANG Xudong, MU Lidan, SUN Tong, AI Ganggang, SHA Yelong, ZHANG Yuqi, WANG Jijiang. Preparation and Application of Triplethiophene Derivative Functionalized SiO2 Inverse Opal Photonic Crystal Fluorescent Films [J]. Chem. J. Chinese Universities, 2021, 42(9): 2989. |
| [11] | YUAN Chunling, YAO Xiaotiao, XU Yuanjin, QIN Xiu, SHI Rui, CHENG Shiqi, WANG Yilin. Colorimetry/Ratio Fluorimetry Determination of Glucose with Bifunctional Carbon Dots [J]. Chem. J. Chinese Universities, 2021, 42(8): 2428. |
| [12] | ZHOU Jieqiong, HUANG Yan, ZHANG Zhiling, PANG Daiwen, TIAN Zhiquan. Water-soluble Ag2Te Quantum Dots with Emission in the Second Near-infrared Window [J]. Chem. J. Chinese Universities, 2021, 42(6): 2072. |
| [13] | LI Xinyu, LI Zhiwei, ZHANG Xingyuan. Construction of Room Temperature Phosphorescence System of Thioflavin-based Polylactide/Benzenesulfonic Acid [J]. Chem. J. Chinese Universities, 2021, 42(6): 1987. |
| [14] | CHEN Hongda, ZHANG Hua, WANG Zhenxin. Development of Small Animals in vivo Fluorescence-photothermal Dual Mode Imaging System [J]. Chem. J. Chinese Universities, 2021, 42(3): 725. |
| [15] | KUANG Xiaojun, YI Jingwei, FANG Xiaoxia, LAI Dongmei, XU Hong. Preparation of Water-soluble Coumarin Fluorescent Substrate and Its Application in Droplet Based Digital Detection [J]. Chem. J. Chinese Universities, 2021, 42(11): 3537. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||