

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (9): 1701.doi: 10.7503/cjcu20160171
• Physical Chemistry • Previous Articles Next Articles
ZONG Meirong1, HE Huichao2, DONG Faqin1,2,*( ), HE Ping2, SUN Shiyong1, LIU Mingxue1, NIE Xiaoqin1
), HE Ping2, SUN Shiyong1, LIU Mingxue1, NIE Xiaoqin1
Received:2016-03-23
Online:2016-09-10
Published:2016-08-26
Contact:
DONG Faqin
E-mail:fqdong@swuat.edu.cn
Supported by:CLC Number:
TrendMD:
ZONG Meirong, HE Huichao, DONG Faqin, HE Ping, SUN Shiyong, LIU Mingxue, NIE Xiaoqin. Electrochemical Electron Transfer and Crystallization Process of Uranium(Ⅵ) in Sodium Salt Solution†[J]. Chem. J. Chinese Universities, 2016, 37(9): 1701.
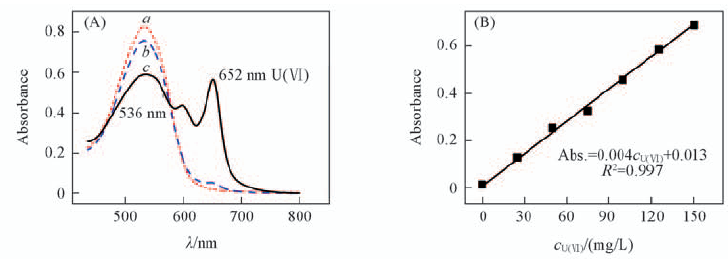
Fig.1 Absorbance spectra of ultra-pure water(a), 0.10 mol/L NaCl(b), 0.10 mol/L NaCl+100 mg/L U(Ⅵ)(c) complexing with arsenazo Ⅲ(A) and linear relationship between Abs. and cU(Ⅵ)(B)
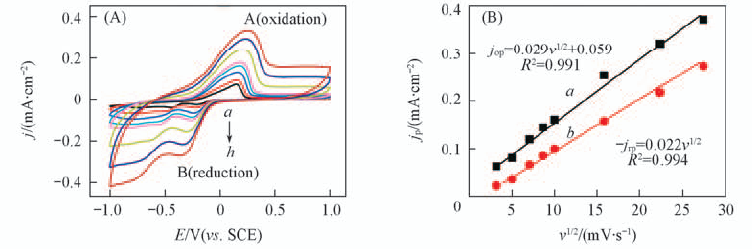
Fig.3 Cyclic voltammogram of FTO electrode in 1 mmol/L U(Ⅵ)+0.1 mol/L NaCl(pH=3.87) at diffe-rent scan rates(A) and linear relationship between jop, -jrp and ν1/2(B) (A) Scan rate/(mV·s-1): a. 10; b. 25; c. 50; d. 75; e. 100; f. 250; g. 500; h. 750. (B) a. for peak A(oxidation); b. for peak B(reduction).
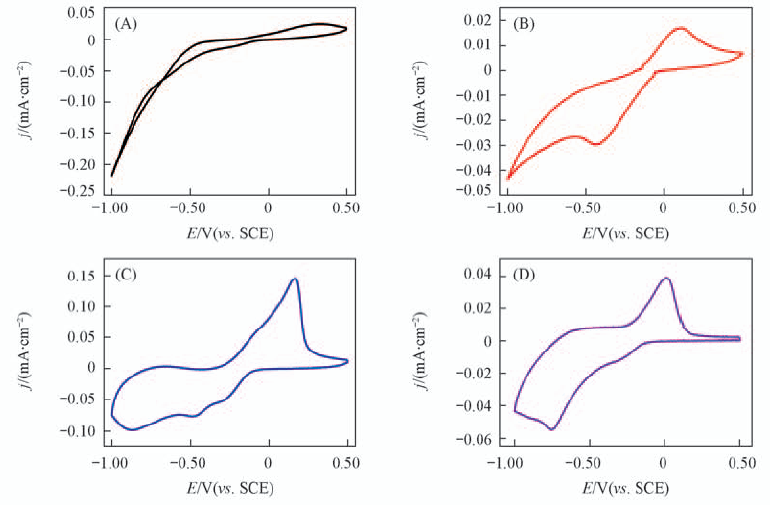
Fig.4 Cyclic voltammogram of FTO electrode in 1.0 mmol/L U(Ⅵ)+0.10 mol/L NaCl solution at pH values of 1.5(A), 3.0(B), 4.5(C) and 6.0(D) The scan rate was 25 mV/s.
| U(Ⅵ) Species | Percent(%) | ||||
|---|---|---|---|---|---|
| pH=1.5 | pH=3.0 | pH=3.87 | pH=4.5 | pH=6 | |
| U | 95.015 | 93.977 | 80.362 | 78.776 | — |
| UO2Cl+ | 4.816 | 5.011 | 4.294 | 4.209 | — |
| UO2OH+ | — | 0.251 | 1.984 | 2.110 | — |
| (UO2)2(OH | — | — | 9.794 | 11.071 | 0.028 |
| (UO2)2OH3+ | 0.015 | 0.452 | 3.043 | 3.171 | — |
| (UO2)3(OH | — | 0.157 | 0.196 | 0.255 | 0.135 |
| (UO2)3OH5+ | — | — | 0.192 | 0.272 | 21.523 |
| (UO2)4OH7+ | — | — | — | — | 78.307 |
| UO2N | 0.131 | 0.137 | 0.117 | 0.115 | — |
| UO2Cl2(aq) | 0.015 | 0.016 | 0.013 | 0.013 | — |
Table 1 Speciation of U(Ⅵ) at different pH conditions calculated by MINTEQ software*
| U(Ⅵ) Species | Percent(%) | ||||
|---|---|---|---|---|---|
| pH=1.5 | pH=3.0 | pH=3.87 | pH=4.5 | pH=6 | |
| U | 95.015 | 93.977 | 80.362 | 78.776 | — |
| UO2Cl+ | 4.816 | 5.011 | 4.294 | 4.209 | — |
| UO2OH+ | — | 0.251 | 1.984 | 2.110 | — |
| (UO2)2(OH | — | — | 9.794 | 11.071 | 0.028 |
| (UO2)2OH3+ | 0.015 | 0.452 | 3.043 | 3.171 | — |
| (UO2)3(OH | — | 0.157 | 0.196 | 0.255 | 0.135 |
| (UO2)3OH5+ | — | — | 0.192 | 0.272 | 21.523 |
| (UO2)4OH7+ | — | — | — | — | 78.307 |
| UO2N | 0.131 | 0.137 | 0.117 | 0.115 | — |
| UO2Cl2(aq) | 0.015 | 0.016 | 0.013 | 0.013 | — |
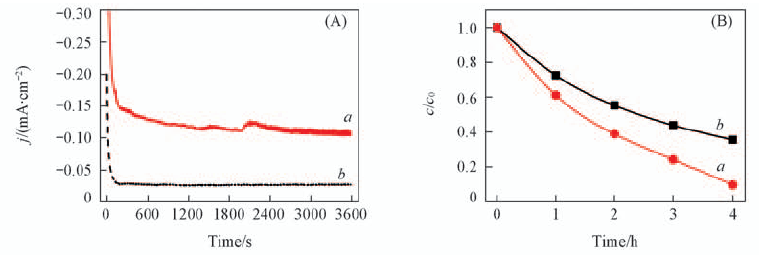
Fig.5 Amperometric i-t curve of FTO electrode in 1 mmol/L U(Ⅵ)+0.1 mol/L NaCl(pH=3.87) at a constant applied potential of -0.50 V(vs. SCE)(A) and variation of the U(Ⅵ) concentration with electrochemical reduction time(B) with(a) or without(b) N2-purging
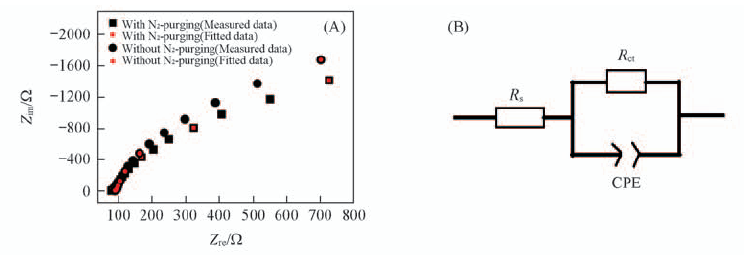
Fig.6 Electrochemical impedance spectra of FTO electrode in 1.0 mmol/L U(Ⅵ)+0.10 mol/L NaCl(pH=3.87) at a constant applied potential of -0.50 V(vs. SCE) with or without N2 purging(A) and equivalent circuit for FTO electrode(B)
| Sample | Rs(Ω)/error(%) | CPE1-T(F)/error(%) | CPE1-P(F)/error(%) | Rct(Ω)/error(%) |
|---|---|---|---|---|
| Without N2-purging | 92.56/0.67 | 5.99×10-5/1.98 | 0.96/0.50 | 6340/6.02 |
| With N2-purging | 84.56/0.98 | 7.10×10-5/2.94 | 0.94/0.75 | 4765/7.57 |
Table 2 Value of the elements in equivalent circuit fitted by the Nyquist plot*
| Sample | Rs(Ω)/error(%) | CPE1-T(F)/error(%) | CPE1-P(F)/error(%) | Rct(Ω)/error(%) |
|---|---|---|---|---|
| Without N2-purging | 92.56/0.67 | 5.99×10-5/1.98 | 0.96/0.50 | 6340/6.02 |
| With N2-purging | 84.56/0.98 | 7.10×10-5/2.94 | 0.94/0.75 | 4765/7.57 |
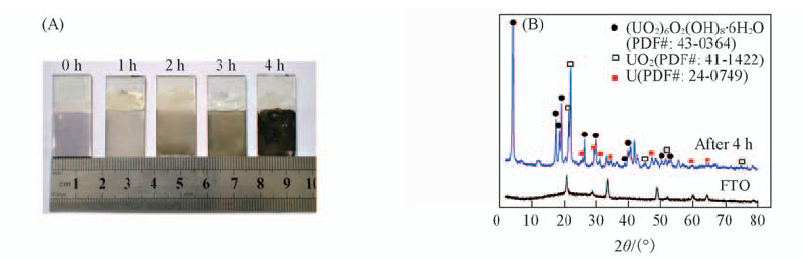
Fig.7 Photographs of FTO electrode after different time i-t measurements in 1.0 mmol/L U(Ⅵ)+0.10 mol/L NaCl(pH=3.87) at a constant applied potential of -0.50 V(vs. SCE) with N2-purging(A) and XRD pattern of FTO electrode before and after electrochemical reduction of U(Ⅵ) solution(B)
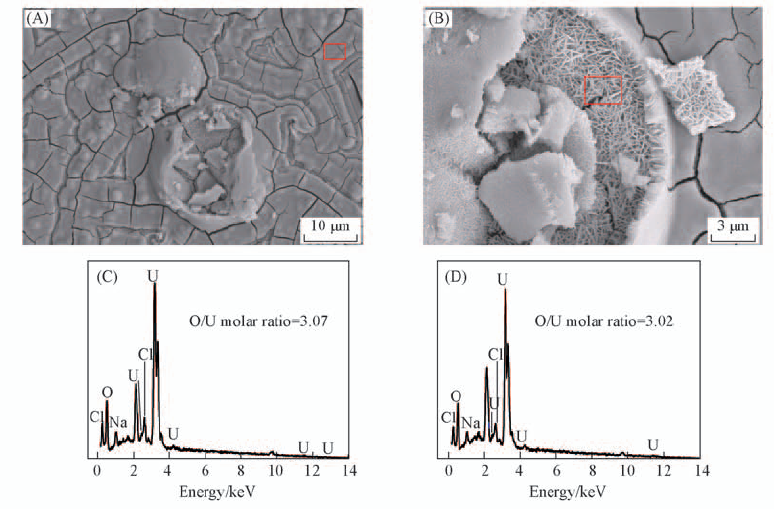
Fig.8 SEM images(A, B) and EDS spectra(C, D) of FTO electrode after electrochemical reduction in U(Ⅵ) solution for 4 h (C) and (D) are the EDS spectra of the framed area in (A) and (B), respectivery.
| [1] | Leggett R. W., Health. Phys., 1989, 57(3), 365—383 |
| [2] | Domingo J. L., Reprod. Toxicol., 2001, 15(6), 603—609 |
| [3] | Sharma P., Tomar R., Micropor. Mesopor. Mater., 2008, 116(1/3), 641—652 |
| [4] | Xie S. B., Yang J., Chen C., Zhang X. J., Wang Q. L., Zhang C., J. Environ. Radioactiv., 2008, 99(1), 126—133 |
| [5] | Xie S. B., Zhang C., Zhou X. H., Yang J., Zhang X. J., Wang J. S., J. Environ. Radioactiv., 2009, 100(2), 162—166 |
| [6] | Ozay O., Ekici S., Aktas N., Sahiner N., J. Environ. Manage,2011, 92(12), 3121—3129 |
| [7] | Li H. Y., Wang B., Liu S. M., Chem. J. Chinese Universities,2015, 36(4), 665—671 |
| (李宏宇, 王博, 刘树明. 高等学校化学学报,2015, 36(4), 665—671) | |
| [8] | Chen L., Li Y., Wang Z. W., Peng Z. Y., Yang Z. M., Yuan L. H., Feng W., Chem. J. Chinese Universities,2015, 36(8), 1485—1490 |
| (陈龙, 李艳, 王真文, 彭智勇, 杨泽明, 袁立华, 冯文. 高等学校化学学报,2015, 36(8), 1485—1490) | |
| [9] | Abbasizadeh S., Keshtkar A. R., Mousavian M. A., Biochem. Eng. J., 2013, 220(15), 161—171 |
| [10] | Sprynskyy M., Kovalchuk I., Buszewski B., J. Hazard. Mater., 2010, 181(1—3), 700—707 |
| [11] | Wang G. H., Liu J. S., Wang X. G., Xie Z. Y., Deng N. S., J. Hazard. Mater., 2009, 168(2/3), 1053—1058 |
| [12] | Wang G. H., Wang X. G., Chai X. J., Liu J. S., Deng N. S., Appl. Clay. Sci., 2010, 47(3/4), 448—451 |
| [13] | Li X. Y., Zhang M., Liu Y. B., Li X., Liu Y. H., Hua R., He C. T., Water. Qual. Expos. Hea,2013, 5(1), 31—40 |
| [14] | Liang F. Y., Wu R. R., Cao C. L., Zheng Y., Yang C. H., Zhao F., Chem. J. Chinese Universities,2014, 35(2), 372—376 |
| (梁方圆, 吴冉冉, 曹昌丽, 郑越, 杨朝晖, 赵峰. 高等学校化学学报,2014, 35(2), 372—376) | |
| [15] | Wei Y. Z., Fang B., Arai T., Kumagai M., J. Radioanal. Nucl. Ch., 2004, 262(2), 409—415 |
| [16] | Anderson C. J., Choppin G. R., Pruett D. J., Costa D., Smith W., Radiochim. Acta,1999, 84(1), 31—36 |
| [17] | Ikeda Y., Hiroe K., Asanuma N., Asanuma N., Shirai A., J. Nucl. Sci. Technol., 2009, 46(2), 158—162 |
| [18] | Zhang Q. Y., Huang X. H., Tang H. B., He H., Journal of Nuclear and Radiochemistry,2011, 33(2), 101—105 |
| (张秋月, 黄小红, 唐洪彬, 何辉. 核化学与放射化学,2011, 33(2), 101—105) | |
| [19] | Wang Y., Liu Y. P., Wang X. Y., Zhu T. W., Journal of Nuclear and Radiochemistry,2013, 35(5), 263—269 |
| (王悦, 刘玉鹏, 王祥云, 褚泰伟. 核化学与放射化学,2013, 35(5), 263—269) | |
| [20] | Tan X. F., Research on the Electrochemical Behavior of Uranium in Ionic Liquids, University of South China, Hengyang, 2014 |
| (谭绪凤. 铀在离子液体中的电化学行为研究, 衡阳: 南华大学, 2014) | |
| [21] | Liu J. A., Feng X. G., Journal of Nuclear and Radiochemistry,2011, 33(1), 32—41 |
| (刘杰安, 冯孝贵. 核化学与放射化学,2011, 33(1), 32—41) | |
| [22] | Qiu L. Y., Yuan L. Y., Tan X. F., Shi W. Q., Liu L. J., Journal of Nuclear and Radiochemistry,2014, 36(2), 65—74 |
| (邱凌云, 袁立永, 谭绪凤, 石伟群, 刘良军. 核化学与放射化学,2014, 36(2), 65—74) | |
| [23] | Yuan K., Ilton E. S., Antonio M. R., Li Z., Cook P. J., Becker U., Environ. Sci. Technol., 2015, 49(10), 6206—6213 |
| [24] | Kim Y. K., Lee S., Ryu. J., Park H., Appl. Catal. B-Environ., 2015, 163(2), 584—590 |
| [25] | Ha D., Jin X., You Kaang Xuan Ye,1981, 4(3), 11—27 |
| (哈迪, 金鑫. 铀矿选冶,1981, 4(3), 11—27) |
| [1] | LI Yidi, TIAN Xiaochun, LI Junpeng, CHEN Lixiang, ZHAO Feng. Electron Transfer on the Semiconductor-microbe Interface and Its Environmental Application [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220089. |
| [2] | LI Yichuan, ZHU Guofu, WANG Yu, CHAI Yongming, LIU Chenguang, HE Shengbao. Effects of Substrate Surface Properties and Precursor Chemical Environment on In⁃situ Oriented Construction of Titanium Silicalite Zeolite Membranes [J]. Chem. J. Chinese Universities, 2021, 42(9): 2934. |
| [3] | LI Yanyan, DUAN Linrui, LUO Jingshan. Moisture-assisted Crystallization of Inorganic Perovskite CsPbI3 Film [J]. Chem. J. Chinese Universities, 2021, 42(6): 1785. |
| [4] | MA Yanrong, JIANG Shengnan, JIN Yan. Sensitive and Electrochemical Detection of Telomerase Activity Based on the Signal Amplification of Strand Displacement Reaction [J]. Chem. J. Chinese Universities, 2021, 42(3): 745. |
| [5] | MIAO Weijun, WU Feng, WANG Yong, WANG Zongbao. In⁃situ Study of the Epitaxial Crystallization of PCL/RGO at High Shear Rate [J]. Chem. J. Chinese Universities, 2021, 42(3): 910. |
| [6] | YANG Pengfei, SHI Yuping, ZHANG Yanfeng. Large-scale Syntheses and Versatile Applications of Two-dimensional Metal Dichalcogenides [J]. Chem. J. Chinese Universities, 2021, 42(2): 504. |
| [7] | WANG Juan, WANG Linying, ZHU Dali, CUI Wenhao, WANG Yifeng, TIAN Peng, LIU Zhongmin. Progress in Direct Synthesis of High Silica Zeolite Y [J]. Chem. J. Chinese Universities, 2021, 42(1): 1. |
| [8] | WU Qinming, WANG Yeqing, MENG Xiangju, XIAO Fengshou. Reconsideration of Crystallization Process for Aluminosilicate Zeolites [J]. Chem. J. Chinese Universities, 2021, 42(1): 21. |
| [9] | WANG Yang, WANG Sidi, TANG Shaokun. Synthesis and Characterization of Imine-based Covalent Organic Framework(COF-LZU1) in Supercritical Carbon Dioxide [J]. Chem. J. Chinese Universities, 2020, 41(8): 1792. |
| [10] | HE Zhechao, XIA Kun, WANG Jing, ZHOU Dan, LU Xinhuan, XIA Qinghua. Controllable Synthesis of SAPO-5 Molecular Sieves and Exploration of the Crystallization Process † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1224. |
| [11] | MAO Qing,ZHAO Jian,LIU Song,GUO Chang,LI Bingyu,XU Keyi,CAO Ziqiang,HUANG Yanqiang. Electrochemical Spectroscopy Analysis for Kinetics of the CO2 Electroreduction Reaction on Ni Single Atom Catalysts † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1058. |
| [12] | HAN Juntian,CUI Yaoxing,SU Zhijun,WU Yi,CHEN Liuping,XU Junhui. Two-Electron Storage Viologen for Aqueous Organic Redox Flow Batteries [J]. Chem. J. Chinese Universities, 2020, 41(5): 1035. |
| [13] | LIU Lu,WU Hanyue,LI Jing,SHE Lan. Tuning Microstructures of Iron-Nickel Alloy Catalysts for Efficient Oxygen Evolution Reaction † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1083. |
| [14] | WANG Yuyao, ZHANG Qiang, YU Jihong. Synthesis of Hierarchical NaX Zeolite and Its CO2 Adsorption Performance † [J]. Chem. J. Chinese Universities, 2020, 41(4): 616. |
| [15] | HAN Fangjie, DAI Mengjiao, LIANG Zhishan, SONG Zhongqian, HAN Dongxue, NIU Li. Research Progress of Photoelectrochemical Technology Applied in Antioxidant Analysis † [J]. Chem. J. Chinese Universities, 2020, 41(4): 591. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||