

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (5): 844.doi: 10.7503/cjcu20150984
• Analytical Chemistry • Previous Articles Next Articles
WANG Xiye1,2, SHAN Xiaotong3, WANG Yilin3, LI Dan1,2, ZHAO Ming3,*( ), XU Liang1,2,*(
), XU Liang1,2,*( )
)
Received:2015-12-26
Online:2016-05-10
Published:2016-04-07
Contact:
ZHAO Ming,XU Liang
E-mail:langzhe73@163.com;nmgxl66@163.com
Supported by:CLC Number:
TrendMD:
WANG Xiye, SHAN Xiaotong, WANG Yilin, LI Dan, ZHAO Ming, XU Liang. Mechanism of Salvia Miltiorrhiza on the Improvement of Myocardial Function in Patients with Dilated Cardiomyopathy†[J]. Chem. J. Chinese Universities, 2016, 37(5): 844.
| Group | LVIDd/mm | LVSd/mm | LVPWDd/mm | LVEF(%) |
|---|---|---|---|---|
| C | 4.73±0.41** | 1.74±0.1** | 2.1±0.33** | 85.5±3.23** |
| M | 5.83±0.59 | 2.08±0.19 | 1.73±0.07 | 70.4±5.69 |
| T | 5.26±0.33** | 1.99±0.18** | 1.91±0.09** | 76.2±3.19** |
Table 1 Effect of salvianolate miltiorrhiza on ventricular structure and ejection fraction in rats with dilated cardiomyopathy
| Group | LVIDd/mm | LVSd/mm | LVPWDd/mm | LVEF(%) |
|---|---|---|---|---|
| C | 4.73±0.41** | 1.74±0.1** | 2.1±0.33** | 85.5±3.23** |
| M | 5.83±0.59 | 2.08±0.19 | 1.73±0.07 | 70.4±5.69 |
| T | 5.26±0.33** | 1.99±0.18** | 1.91±0.09** | 76.2±3.19** |
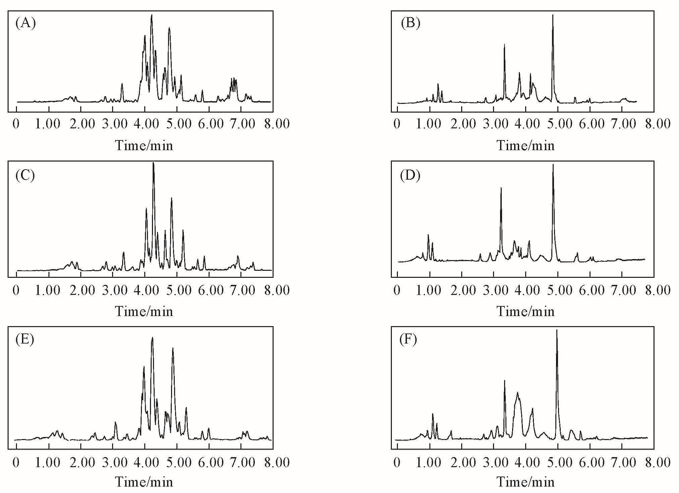
Fig.2 Base peak intensity chromatograms(BPI) under the ESI+ mode(A, C, E) and ESI- mode(B, D, F) (A), (B) Control group; (C), (D) model group; (E), (F) treatment group.
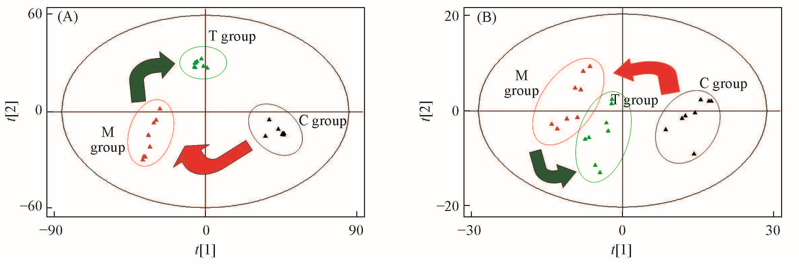
Fig.3 PCA score plots obtained from the metabolic profiles of the control group, model group and treatment group under positive(A) and negative ion modes(B)
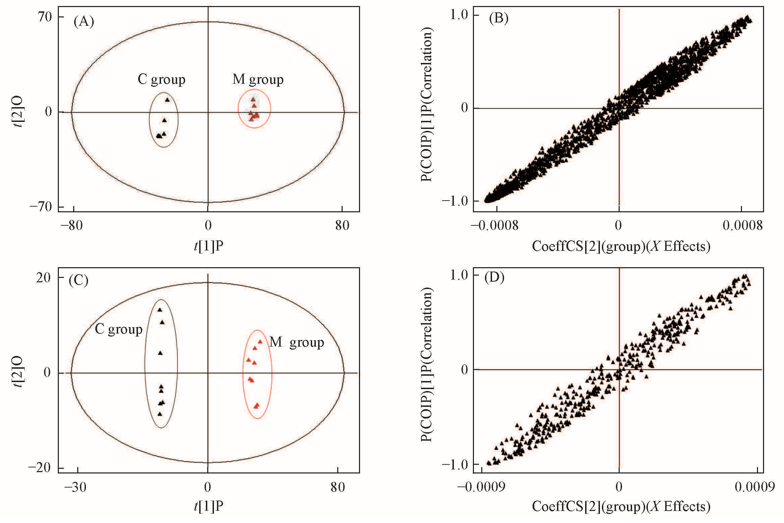
Fig.4 OPLS-DA score plots(A, C) and S-plots(B, D) obtained from the metabolic profiles of the control group and model group under positive(A, B) and negative ion modes(C, D)
| tR/min | Measured mass | 106Mass deviation | MS2, m/z | Chemical formula | Compound | ESI mode |
|---|---|---|---|---|---|---|
| 6.89 | 762.5300 | 2.6 | 745.5, 265.3, 239.2, 88.0 | C40H76NO10P | PS[16∶0/18∶1(9Z)] | + |
| 4.56 | 496.3406 | 1.6 | 330.1 | C24H50NO7P | LysoPC(16∶0) | + |
| 4.44 | 544.3402 | 0.7 | 378.2 | C28H50NO7P | LysoPC[20∶4 | + |
| (5Z,8Z,11Z,14Z)] | ||||||
| 4.45 | 568.3394 | 0.7 | 402.7 | C30H50NO7P | LysoPC[22∶6 | + |
| (4Z,7Z,10Z,13Z,16Z,19Z)] | ||||||
| 5.97 | 465.3038 | 1.3 | 97.1 | C27H46O4S | Cholesterol sulfate | - |
| 3.66 | 514.2833 | 2.1 | 498.5, 480.6, 462.6 | C26H45NO7S | Taurohyocholate | - |
| 4.28 | 277.2167 | 2.2 | 93.2, 79.4, 67.3, 55.6 | C18H30O2 | Gamma-Linolenic acid | - |
| 4.64 | 329.2481 | 1.5 | 271.2 | C22H34O2 | Docosapentaenoic acid | - |
| 4.96 | 375.2533 | 2.1 | 344.2, 288.6, 275.3, 101.5 | C23H36O4 | 9'-Carboxy-γ-chromanol | - |
Table 2 Potential biomarkers between control group and model group
| tR/min | Measured mass | 106Mass deviation | MS2, m/z | Chemical formula | Compound | ESI mode |
|---|---|---|---|---|---|---|
| 6.89 | 762.5300 | 2.6 | 745.5, 265.3, 239.2, 88.0 | C40H76NO10P | PS[16∶0/18∶1(9Z)] | + |
| 4.56 | 496.3406 | 1.6 | 330.1 | C24H50NO7P | LysoPC(16∶0) | + |
| 4.44 | 544.3402 | 0.7 | 378.2 | C28H50NO7P | LysoPC[20∶4 | + |
| (5Z,8Z,11Z,14Z)] | ||||||
| 4.45 | 568.3394 | 0.7 | 402.7 | C30H50NO7P | LysoPC[22∶6 | + |
| (4Z,7Z,10Z,13Z,16Z,19Z)] | ||||||
| 5.97 | 465.3038 | 1.3 | 97.1 | C27H46O4S | Cholesterol sulfate | - |
| 3.66 | 514.2833 | 2.1 | 498.5, 480.6, 462.6 | C26H45NO7S | Taurohyocholate | - |
| 4.28 | 277.2167 | 2.2 | 93.2, 79.4, 67.3, 55.6 | C18H30O2 | Gamma-Linolenic acid | - |
| 4.64 | 329.2481 | 1.5 | 271.2 | C22H34O2 | Docosapentaenoic acid | - |
| 4.96 | 375.2533 | 2.1 | 344.2, 288.6, 275.3, 101.5 | C23H36O4 | 9'-Carboxy-γ-chromanol | - |
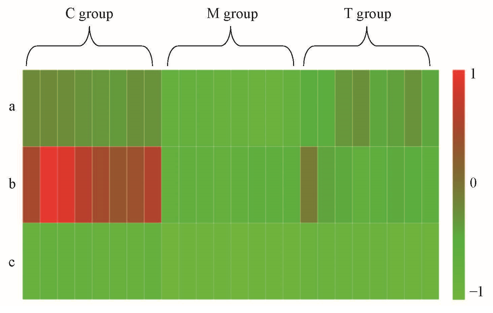
Fig.5 Heatmap of metabolites between control(C) group, model(M) group and treatment(T) groupa. γ-linolenic acid; b. docosapentaenoic acid; c. 9'-carboxy-γ-chromanol.
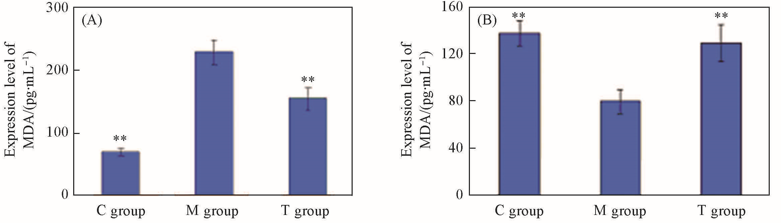
Fig.6 Expression levels of MDA(A) and SOD(B) protein of rats left ventricular myocardial tissue in control group, model group and treatment group*P<0.05, **P<0.01(vs. model group).
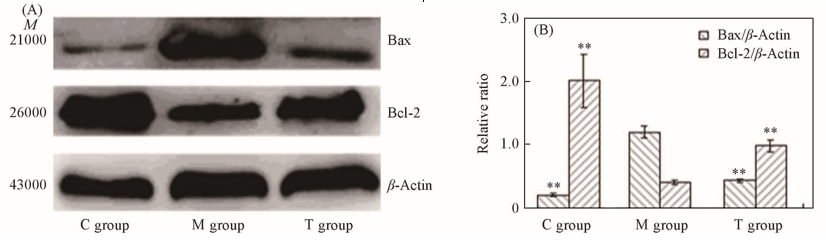
Fig.7 Western blot analysis(A) and expression levels(B) of Bcl-2 and Bax protein of rats left ventricular myocardial tissue in control group, model group and treatment group**P<0.01(vs. model group).
| [1] | Quiles J. L., Huertas J. R., Battino M., Mataix J., Toxicology,2002, 180, 79—95 |
| [2] | Han Y. M., Oh H., Na M., Kim B.Y., Jeong D. G., Ryu S. E., Sok D. E., Ahn J. S., Biol. Pharm.Bull., 2005, 28(9), 1795—1797 |
| [3] | Yang Z.X., Lin Q., Ma L., World Journal of Integrated Traditioal and WesternMedicine, 2012, 7(2), 93—96 |
| (杨志霞, 林谦, 马利. 世界中西医结合杂志, 2012, 7(2), 93—96) | |
| [4] | Chen C., Zhou X. G., Qiu S. D., Chen H., Chen Y. Z., Lin X. M., Chinese Journal of Integrated Traditional and WesternMedicine, 2015,35(7), 871—876 |
| (陈成, 邹襄谷, 邱山东, 陈慧, 陈永忠, 林秀明. 中国中西医结合杂志, 2015, 35(7), 871—876) | |
| [5] | Fang L. Y., Liu Y. Y., Li G. D., Ling Y., Liang Y. B., Deng Y. L., Yu L., Int. J. Cardiovasc.Dis., 2015, 42(4), 256—260 |
| (方凌燕, 刘衍宇, 李国达, 凌云, 梁一波, 邓玉丽, 余丽. 国际心血管病杂志, 2015, 42(4), 256—260) | |
| [6] | Zhang D. F., Wang M. W., Wang L. S., Tang J. J., Chen B., Wang W., Yang Z. J., Cao K. J., Chinese Journal of Integrative Medicine on Cardi-/Cerebrovascular Disease, 2008, 6(11), 1304—1306 |
| (张殿福, 王明伟, 王连生, 唐建金, 陈波, 王伟, 杨志健, 曹克将. 中西医结合心脑血管病杂志, 2008, 6(11), 1304—1306) | |
| [7] | Wang Y. B., Xiao D., Li X. Y., Dai Y. L., Yue H., Liu S. Y., Chem. J. Chinese Universities,2015, 36(10), 1894—1899 |
| (王一博, 肖丹, 李晓宇, 戴雨霖, 越皓, 刘淑莹. 高等学校化学学报, 2015, 36(10), 1894—1899) | |
| [8] | Zhang R. X., Liu S., Pi Z. F., Song F. R., Liu Z. Q., Chem. J. Chinese Universities,2014, 35(6), 1146—1151 |
| (张瑞兴, 刘舒, 皮子凤, 宋凤瑞, 刘志强. 高等学校化学学报, 2014, 35(6), 1146—1151) | |
| [9] | Dong J., Cai X. M., Zou L. J., Chen C., Xue X. Y., Zhang X. L., Liang X. M., Chem. Res. ChineseUniversities, 2011, 27(5), 750—755 |
| [10] | Zhou R., Yang C. H., Wang Q., Wang F. Z., Chinese Journal of Integrative Medicine on Cardi-/Cerebrovascular Disease, 2009, 7(11), 1315—1317 |
| (周荣, 杨彩虹, 王强, 王凤芝. 中西医结合心脑血管病杂志, 2009, 7(11), 1315—1317) | |
| [11] | Zhao H., Li H.T., Gu D.W., Li S.X., Fang Y.S., Han C.P., Tianjin Med.J., 2012, 40(1), 64—66 |
| (赵红, 李海涛, 顾定伟, 李素新, 方艳淑, 韩春萍. 天津医药, 2012, 40(1), 64—66) | |
| [12] | Deng W. K., Wang Y. B., Liu Z. X., PlosOne, 2014, 9(11), e111988[2014-11-05].doi:10.1371/journal.pone.0111988 |
| [13] | Jiang Q., Yin X. M., Lill M. A., Danielson M. L., Danielson M. L., Freiser H., Huang J. J., PNAS, 2015, 105(51), 20464—20469 |
| [14] | Kamal A. A., Khalid S. H., BMC Vet.Res., 2012, 8, 45—52 |
| [15] | Ali S. F., Woodman O. L., Oxid. Med. CellLongev., 2015, e150829[2015-05-17]. doi: 10.1155/2015/150829 |
| [16] | Liu W. N., Leung K. N., PlosOne, 2015, 10(12), e0143684[2015-12-02]. doi: 10.1371/journal.pone.0143684 |
| [17] | Dong J. M., Wu R. H., Yuan C. L., Zhou L. J., Journal of HygieneResearch, 2003, 32(3), 299—301 |
| (董杰明, 吴瑞华, 袁昌鲁, 周连甲. 卫生研究, 2003, 32(3), 299—301) | |
| [18] | Chanikul C., Sukanya J., Sarocha P., Pattsarun C., Sarinya S., Mayura V., Kobkul L., J.Biotechnol., 2016, 218(20), 85—93 |
| [19] | Zhu N., William E., Gany Y., Chin. J.Cardiol., 1998, 26(1), 12—14 |
| [20] | Huang M. F., Wu G. P., Jiao B. N., Food and Drug, 2007, 9(2), 69—71 |
| (黄明发, 吴桂苹, 焦必宁. 食品与药品, 2007, 9(2), 69—71) | |
| [21] | Rachel A. M., Elaine A. Y., Eric D. C., Saurabh M., Michaell M. B., Nutrients, 2015, 7(12), 10282—10289 |
| [22] | Yang Q. H., Tang S. S., Chin.Pharm., 2009, 20(30), 2336—2337 |
| (杨群华, 唐省三. 中国药房, 2009, 20(30), 2336—2337) | |
| [23] | Zhou S. X., Zhang Y. L., Zhou Y., Lei J., Chin. J.Hypertension, 2008, 16(10), 907—911 |
| (周淑娴, 张玉玲, 周艳, 雷娟. 中华高血压杂志, 2008, 16(10), 907—911) | |
| [24] | Du B., Chen W., Chen J. L., Pang Q. F., Chinese Journal of Hospital Pharmacy, 2015, 35(23), 2071—2074 |
| (杜斌, 陈炜, 陈俊良, 庞庆丰. 中国医院药学杂志, 2015, 35(23), 2071—2074) | |
| [25] | Butinar B., Bucar M. M., Mariani C., Raspor P., FoodChem., 2011, 128(2), 505—512 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||