

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (3): 480.doi: 10.7503/cjcu20150835
• Organic Chemistry • Previous Articles Next Articles
LIN Hai1, LI Yawei2, LIN Huakuan2,*( )
)
Received:2015-10-30
Online:2016-03-10
Published:2016-01-30
Contact:
LIN Huakuan
E-mail:hklin@nankai.edu.cn
Supported by:CLC Number:
TrendMD:
LIN Hai, LI Yawei, LIN Huakuan. Anion Recognition of Indole-3-Aldehyde-o-Nitrophenylsemicarbazone†[J]. Chem. J. Chinese Universities, 2016, 37(3): 480.
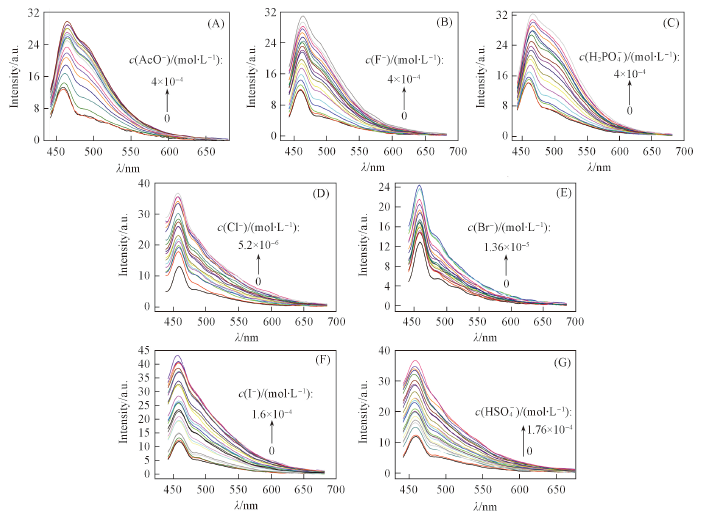
Fig.2 Fluorescence spectrum changes of receptor 1(4.0×10-5 mol/L) in DMSO with increasing concentration of AcO-(A), F-(B), H2PO4-(C), Cl-(D), Br-(E), I-(F) and HSO4-(G) λex=404 nm. Concentration step/(μmol·L-1): (A)—(C) 22.2; (D) 1.1; (E) 6.0; (F) 18.8; (G) 80.
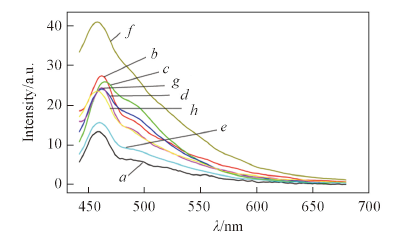
Fig.3 Fluorescence spectra of 4.0×10-5 mol/L receptor 1(a) in DMSO with addition of 2×10-4 mol/L AcO-(b), F-(c), H2PO4-(d), HSO4-(e), 5.2×10-6 mol/L Cl-(f), 1.36×10-5 mol/L Br-(g) and 1.6×10-4 mol/L I-(h), respectively
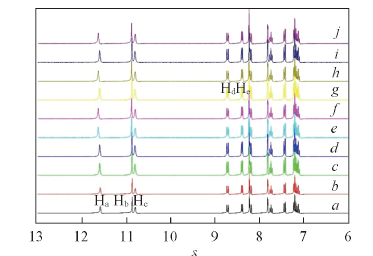
Fig.5 1H NMR titration of 0.01 mol/L receptor (DMSO-d6)with [Bu4N]Clc(Cl-)/(mol·L-1) from a to j: 0, 0.0001, 0.0003, 0.0005, 0.0007, 0.0010, 0.0025, 0.0060, 0.0260, 0.1060.
| Anion | lgKass | Association ratio* | Anion | lgKass | Association ratio* |
|---|---|---|---|---|---|
| AcO- | 6.98 | 1∶2 | Br- | 6.34 | 1∶1 |
| F- | 6.85 | 1∶2 | I- | 5.40 | 1∶1 |
| H2P | 5.40 | 1∶2 | HS | 2.88 | 1∶1 |
| Cl- | 13.51 | 1∶2 |
Table 1 Association constants and ratios for various anions toward receptor
| Anion | lgKass | Association ratio* | Anion | lgKass | Association ratio* |
|---|---|---|---|---|---|
| AcO- | 6.98 | 1∶2 | Br- | 6.34 | 1∶1 |
| F- | 6.85 | 1∶2 | I- | 5.40 | 1∶1 |
| H2P | 5.40 | 1∶2 | HS | 2.88 | 1∶1 |
| Cl- | 13.51 | 1∶2 |
| [1] | Crooks P. A., Ravard A., Wilkins L. H., Teng L. H., Buxton S. T., Dwoskin L. P., Drug Development Research, 1995, 36(2), 91—102 |
| [2] | Samet M., Danesh-Yazdi M., Fattahi A., Kass S. R., J. Org. Chem., 2015, 80(2), 1130—1135 |
| [3] | Robinson S. W., Mustoe C. L., White N. G., Brown A., Thompson A. L., Kennepohl P., Beer P. D., J. Am. Chem. Soc., 2015, 137(1), 499—507 |
| [4] | Liu Y. Z., Yuan K., Lv L. L., Zhu Y. C., Yuan Z., J. Phys. Chem. A, 2015, 119(22), 5842—5852 |
| [5] | Martínez-Aguirre M. A., Yatsimirsky A. K., J. Org. Chem., 2015, 80(10), 4985—4993 |
| [6] | Yang Y. P., Chen S. Y., Ni X. L., J. Anal. Chem., 2015, 87(14), 7461—7466 |
| [7] | Mirza-Aghayan M., Yarmohammadi M., Mohammadian N., Zadmard R., Asadi F., J. Incl. Phenom. Macrocycl. Chem., 2015, 83(1/2), 53—61 |
| [8] | Zhuo J. B., Wan Q., Yan X. Q., Xie L. L., Yuan Y. F., Chem. J. Chinese Universities, 2015, 36(3), 477—483 |
| (卓继斌, 万倩, 宴希泉, 谢莉莉, 袁耀锋. 高等学校化学学报, 2015, 36(3), 477—483) | |
| [9] | Dong Z. Y., Jiang X. Z., Zhang D. W., Gao G. H., Chem. J. Chinese Universities, 2012, 33(10), 2256—2262 |
| (董智云, 江小枝, 张大卫, 高国华. 高等学校化学学报, 2012, 33(10), 2256—2262) | |
| [10] | Boiocchi M., Licchelli M., Milani M., Poggi A., Sacchi D., Inorg. Chem., 2015, 54(1), 47—58 |
| [11] | Chen S. Q., Huang X. H., Technology & Development of Chemical Industry,2009, 38(5), 5—7 |
| (陈素琴, 黄向红. 化工技术与开发, 2009, 38(5), 5—7) | |
| [12] | Wang X. H., He Y. N., Pan M. D., Lin Q., Applied Chemical Industry, 2008, 37(9), 1019—1021 |
| (王向辉, 贺永宁, 盘茂东, 林强. 应用化工, 2008, 37(9), 1019—1021) | |
| [13] | Yu Y., Lin L. R., Yang K. B., Zhang H., Huang R. B., Zheng L. S., Chinese Journal of Organic Chemistry,2006, 26(7), 966—936 |
| (俞芸, 林丽榕, 杨开冰, 章慧, 黄荣彬, 郑兰荪. 有机化学,2006, 26(7), 933—936) | |
| [14] | Connors K., Binding Constants: the Measurement of Molecular Complex Stability, Wiley, New York, 1987, 25—28 |
| [1] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [2] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [3] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [4] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [5] | WU Zexin, ZHU Yuanjie, WANG Hongzhong, WANG Junan, HE Ying. Methyl-modified Carbazole/Diphenyl Sulfone-based AIE-TADF Blue Emitter and Its OLEDs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220371. |
| [6] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [7] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [8] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [9] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [10] | LI Ran, ZHANG Xudong, MU Lidan, SUN Tong, AI Ganggang, SHA Yelong, ZHANG Yuqi, WANG Jijiang. Preparation and Application of Triplethiophene Derivative Functionalized SiO2 Inverse Opal Photonic Crystal Fluorescent Films [J]. Chem. J. Chinese Universities, 2021, 42(9): 2989. |
| [11] | YUAN Chunling, YAO Xiaotiao, XU Yuanjin, QIN Xiu, SHI Rui, CHENG Shiqi, WANG Yilin. Colorimetry/Ratio Fluorimetry Determination of Glucose with Bifunctional Carbon Dots [J]. Chem. J. Chinese Universities, 2021, 42(8): 2428. |
| [12] | ZHOU Jieqiong, HUANG Yan, ZHANG Zhiling, PANG Daiwen, TIAN Zhiquan. Water-soluble Ag2Te Quantum Dots with Emission in the Second Near-infrared Window [J]. Chem. J. Chinese Universities, 2021, 42(6): 2072. |
| [13] | CHEN Hongda, ZHANG Hua, WANG Zhenxin. Development of Small Animals in vivo Fluorescence-photothermal Dual Mode Imaging System [J]. Chem. J. Chinese Universities, 2021, 42(3): 725. |
| [14] | XI Jing, CHEN Na, YANG Yanbing, YUAN Quan. Recent Progress in Controlled Synthesis of Persistent Luminescence Nanomaterials for Diagnosis Applications [J]. Chem. J. Chinese Universities, 2021, 42(11): 3247. |
| [15] | KUANG Xiaojun, YI Jingwei, FANG Xiaoxia, LAI Dongmei, XU Hong. Preparation of Water-soluble Coumarin Fluorescent Substrate and Its Application in Droplet Based Digital Detection [J]. Chem. J. Chinese Universities, 2021, 42(11): 3537. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||