

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (12): 2421.doi: 10.7503/cjcu20150445
• Organic Chemistry • Previous Articles Next Articles
XU Hai*( ), ZHAO Siqi, REN Yang, CAI Jianfeng, WANG Xiang, LIN Yulin
), ZHAO Siqi, REN Yang, CAI Jianfeng, WANG Xiang, LIN Yulin
Received:2015-06-08
Online:2015-12-10
Published:2015-10-10
Contact:
XU Hai
E-mail:xhisaac@csu.edu.cn
Supported by:CLC Number:
TrendMD:
XU Hai, ZHAO Siqi, REN Yang, CAI Jianfeng, WANG Xiang, LIN Yulin. Bridged Di-py-cavitand Compounds as Molecular Switch†[J]. Chem. J. Chinese Universities, 2015, 36(12): 2421.
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M+H]+ | IR(KBr), |
|---|---|---|---|---|---|
| 3 | Yellow solid | 100 | 194—195 | 498.1804 | 3039, 2920, 2851, 2360, 2206, 2106, 1922, 1794, 1738, 1674, |
| (498.1795) | 1600, 1516, 1463, 1435, 1407, 1298, 1243, 1181, 1103, 1018, | ||||
| 971, 836, 757, 716, 681, 647, 613 | |||||
| 4 | Green solid | 100 | 228—229 | 426.1409 | 3272, 3036, 2360, 2207, 2105, 1917, 1794, 1662, 1598, 1516, |
| (426.1398) | 1406, 1264, 1104, 965, 899, 832, 761, 717, 661, 632 | ||||
| 6a | Yellow solid | 34 | >300 | 2022.7986 | 3044, 2924, 2852, 2202, 1738, 1583, 1483, 1412, 1361, 1260, |
| (2022.7934) | 1157, 1108, 843, 800, 752 | ||||
| 6b | Yellow solid | 17 | >300 | 2422.9210 | 3038, 2955, 2922, 2852, 2360, 2210, 2160, 1738, 1600, 1482, |
| (2422.9238) | 1412, 1364, 1260, 1202, 1158, 1068, 840, 763, 717 |
Table 1 Appearance, yields, melting points, HRMS and IR data of compounds 3, 4, 6a and 6b
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M+H]+ | IR(KBr), |
|---|---|---|---|---|---|
| 3 | Yellow solid | 100 | 194—195 | 498.1804 | 3039, 2920, 2851, 2360, 2206, 2106, 1922, 1794, 1738, 1674, |
| (498.1795) | 1600, 1516, 1463, 1435, 1407, 1298, 1243, 1181, 1103, 1018, | ||||
| 971, 836, 757, 716, 681, 647, 613 | |||||
| 4 | Green solid | 100 | 228—229 | 426.1409 | 3272, 3036, 2360, 2207, 2105, 1917, 1794, 1662, 1598, 1516, |
| (426.1398) | 1406, 1264, 1104, 965, 899, 832, 761, 717, 661, 632 | ||||
| 6a | Yellow solid | 34 | >300 | 2022.7986 | 3044, 2924, 2852, 2202, 1738, 1583, 1483, 1412, 1361, 1260, |
| (2022.7934) | 1157, 1108, 843, 800, 752 | ||||
| 6b | Yellow solid | 17 | >300 | 2422.9210 | 3038, 2955, 2922, 2852, 2360, 2210, 2160, 1738, 1600, 1482, |
| (2422.9238) | 1412, 1364, 1260, 1202, 1158, 1068, 840, 763, 717 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(75 MHz, CDCl3), δ |
|---|---|---|
| 2 | 0.26(s, 9H, CH3), 7.24(m, 2H, apparent AA'BB' ArH), 7.44(s, 1H, ArH), 7.79(m, 2H, apparent AA'BB' ArH) | 0.04, 90.30, 94.36, 96.43, 104.44, 122.39, 122.81, 123.07, 128.34, 131.24, 131.80, 132.93, 137.43 |
| 3 | 0.26(s, 9H, CH3), 7.58—7.78(q, 4H, ArH), 7.50(s, 4H, ArH), 8.02—8.75(m, 9H, PyH) | 0.04, 90.30, 94.36, 96.43, 104.44, 122.39, 122.81, 123.07, 128.34, 131.24, 131.80, 132.93, 137.43 |
| 4 | 3.20(s, 1H, CH), 7.58—7.73(q, 4H, J=8.4 Hz, ArH), 7.50(d, 4H, ArH), 8.04—8.67(m, 9H, J=0.9 Hz, PyH) | 132.00, 131.83, 131.57, 131.52, 131.39, 131.14, 130.94, 129.54, 128.26, 128.21, 127.14, 126.19, 125.62, 125.58, 125.34, 124.46, 123.54, 123.42, 122.70, 121.99, 117.33, 94.74, 91.11, 90.75, 83.21, 79.03 |
| 6a | 0.91—0.97(m, 12H, CH3), 1.24—1.59(m, 40H), 2.05—2.30(m, 12H), 5.60—5.81(m, 4H, methane CH) 7.36(s, 4H ArH), 7.43—7.45(q, 4H, J=3.4 Hz, ArH), 7.58(d, 4H, J=9.0 Hz, ArH) 8.15(s, 4H, ArH), 7.64—8.45(m, 22H, ArH and PyH) | 14.25, 18.34, 18.49, 22.85, 28.13, 29.55, 29.88, 32.06, 32.37, 32.81, 34.35, 90.50, 94.13, 117.23, 119.02, 123.97, 12.24, 124.50, 124.64, 125.33, 125.68, 125.87, 125.95, 126.25, 127.23, 128.59, 128.66, 128.97, 129.83, 130.96, 131.18, 131.63, 132.14, 132.385, 135.91, 136.32, 137.06, 137.71, 140.00, 141.74, 152.11, 152.27, 152.48, 153.27, 159.06, 161.69 |
| 6b | 0.91—0.97(m, 12H, CH3), 1.24—1.56(m, 40H), 2.05—2.30(m, 12H), 5.60—5.81(m, 4H, methane CH), 7.27(s, 4H, ArH), 7.49(s, 4H, ArH), 7.30—7.34(q, 4H, J=3.3 Hz, ArH), 7.61(s, 8H, ArH), 7.64—8.45(m, 16H, PyH), 8.20—8.30(m, 16H, ArH), 8.66—8.70(d, 2H, J=9.3 Hz, PyH) | 14.26, 14.31, 17.87, 17.99, 18.22, 18.29, 22.86, 28.14, 28.17, 29.56, 29.91, 32.07, 32.37, 32.89, 34.39, 34.49, 90.50, 90.82, 91.05, 91.24, 91.57, 95.05, 96.72, 117.70, 119.00, 123.10, 123.60, 123.97, 124.58, 124.80, 125.40, 125.70, 125.97, 126.53, 127.49, 128.54, 128.61, 128.71, 128.98, 129.13, 129.61, 129.90, 131.32, 131.51, 131.63, 131.71, 131.89, 131.92, 131.95, 131.98, 132.22, 132.43, 135.92, 136.21, 137.06, 137.09, 137.61, 138.04, 138.43, 139.34, 140.02, 141.63, 141.70, 141.76, 152.24, 152.26, 152.45, 153.30, 159.08, 161.51, 161.64 |
Table 2 1H NMR and 13C NMR data of compounds 2—4, 6a, 6b
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(75 MHz, CDCl3), δ |
|---|---|---|
| 2 | 0.26(s, 9H, CH3), 7.24(m, 2H, apparent AA'BB' ArH), 7.44(s, 1H, ArH), 7.79(m, 2H, apparent AA'BB' ArH) | 0.04, 90.30, 94.36, 96.43, 104.44, 122.39, 122.81, 123.07, 128.34, 131.24, 131.80, 132.93, 137.43 |
| 3 | 0.26(s, 9H, CH3), 7.58—7.78(q, 4H, ArH), 7.50(s, 4H, ArH), 8.02—8.75(m, 9H, PyH) | 0.04, 90.30, 94.36, 96.43, 104.44, 122.39, 122.81, 123.07, 128.34, 131.24, 131.80, 132.93, 137.43 |
| 4 | 3.20(s, 1H, CH), 7.58—7.73(q, 4H, J=8.4 Hz, ArH), 7.50(d, 4H, ArH), 8.04—8.67(m, 9H, J=0.9 Hz, PyH) | 132.00, 131.83, 131.57, 131.52, 131.39, 131.14, 130.94, 129.54, 128.26, 128.21, 127.14, 126.19, 125.62, 125.58, 125.34, 124.46, 123.54, 123.42, 122.70, 121.99, 117.33, 94.74, 91.11, 90.75, 83.21, 79.03 |
| 6a | 0.91—0.97(m, 12H, CH3), 1.24—1.59(m, 40H), 2.05—2.30(m, 12H), 5.60—5.81(m, 4H, methane CH) 7.36(s, 4H ArH), 7.43—7.45(q, 4H, J=3.4 Hz, ArH), 7.58(d, 4H, J=9.0 Hz, ArH) 8.15(s, 4H, ArH), 7.64—8.45(m, 22H, ArH and PyH) | 14.25, 18.34, 18.49, 22.85, 28.13, 29.55, 29.88, 32.06, 32.37, 32.81, 34.35, 90.50, 94.13, 117.23, 119.02, 123.97, 12.24, 124.50, 124.64, 125.33, 125.68, 125.87, 125.95, 126.25, 127.23, 128.59, 128.66, 128.97, 129.83, 130.96, 131.18, 131.63, 132.14, 132.385, 135.91, 136.32, 137.06, 137.71, 140.00, 141.74, 152.11, 152.27, 152.48, 153.27, 159.06, 161.69 |
| 6b | 0.91—0.97(m, 12H, CH3), 1.24—1.56(m, 40H), 2.05—2.30(m, 12H), 5.60—5.81(m, 4H, methane CH), 7.27(s, 4H, ArH), 7.49(s, 4H, ArH), 7.30—7.34(q, 4H, J=3.3 Hz, ArH), 7.61(s, 8H, ArH), 7.64—8.45(m, 16H, PyH), 8.20—8.30(m, 16H, ArH), 8.66—8.70(d, 2H, J=9.3 Hz, PyH) | 14.26, 14.31, 17.87, 17.99, 18.22, 18.29, 22.86, 28.14, 28.17, 29.56, 29.91, 32.07, 32.37, 32.89, 34.39, 34.49, 90.50, 90.82, 91.05, 91.24, 91.57, 95.05, 96.72, 117.70, 119.00, 123.10, 123.60, 123.97, 124.58, 124.80, 125.40, 125.70, 125.97, 126.53, 127.49, 128.54, 128.61, 128.71, 128.98, 129.13, 129.61, 129.90, 131.32, 131.51, 131.63, 131.71, 131.89, 131.92, 131.95, 131.98, 132.22, 132.43, 135.92, 136.21, 137.06, 137.09, 137.61, 138.04, 138.43, 139.34, 140.02, 141.63, 141.70, 141.76, 152.24, 152.26, 152.45, 153.30, 159.08, 161.51, 161.64 |
| Compound 6a | Compound 6b | |||||||
|---|---|---|---|---|---|---|---|---|
| System | Pyrene shift | T/℃ | Pyrene shift | System | Pyrene shift | T/℃ | Pyrene shift | |
| NaOD | 8.46 | 35 | 8.47 | NaOD | 8.69 | 35 | 8.69 | |
| TFA-D | 8.67 | -20 | 8.47 | TFA-D | 8.69 | -20 | 8.68 | |
| D2O | 8.46 | -65 | 8.69 | D2O | 8.72 | -65 | 8.67 | |
Table 3 Chemical shift of di-py-cavitand compounds
| Compound 6a | Compound 6b | |||||||
|---|---|---|---|---|---|---|---|---|
| System | Pyrene shift | T/℃ | Pyrene shift | System | Pyrene shift | T/℃ | Pyrene shift | |
| NaOD | 8.46 | 35 | 8.47 | NaOD | 8.69 | 35 | 8.69 | |
| TFA-D | 8.67 | -20 | 8.47 | TFA-D | 8.69 | -20 | 8.68 | |
| D2O | 8.46 | -65 | 8.69 | D2O | 8.72 | -65 | 8.67 | |
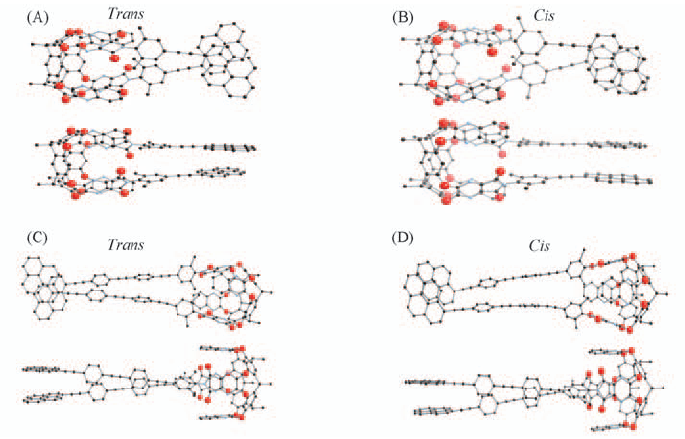
Fig.3 Cis/trans isomer of structures of bridged di-py-cavtiand compounds with “bud” conformation (A) Dcc=0; (B) Dcc=0.39 nm; (C) Dcc=0.38 nm; (D) Dcc=0.38 nm.
| [1] | Schrader T., Hamilton A.D., Functional Synthetic Receptors, John Wiley & Sons, Weinheim, 2006, 257 |
| [2] | Ramamurthy V., Schanze K.S., Optical Sensors and Switches, CRC Press, Boca Raton, 2001, 25—26 |
| [3] | Balzani V., Credi A., Raymo F. M., Stoddart J. F., Angew. Chem. Int. Ed., 2000, 39(19), 3348—3391 |
| [4] | Taylor R. J., Tetrahedron, 2008, 64 (36), 8229 |
| [5] | Li L., Yan Y., Wang D. M., Zheng D. F., Li J. Y., Chem. J. Chinese. Universities,2014, 35(11), 2285—2290 |
| (李莉, 颜岩, 王大明, 郑大方, 李激扬. 高等学校化学学报,2014, 35(11), 2285—2290) | |
| [6] | Kay E. R., Leigh D. A., Zerbetto F., Angew. Chem. Int. Ed., 2007, 46 (1/2), 72—191 |
| [7] | Browne W. R., Feringa B. L., Nat. Nanotech., 2006, 1(1), 25—35 |
| [8] | Beckman R., Beverly K., Boukai A., Bunimovich Y., Choi J. W., DeIonno E., Green J., Johnston-Halperin E., Luo Y., Sheriff B., Faraday Discuss., 2006, 131, 9—22 |
| [9] | Chen H., Jia X., Li C., Chin. J. Chem., 2015, 33(3), 343—345 |
| [10] | Song N., Yang Y. W., Chem. Soc. Rev., 2015, 44, 3474—3504 |
| [11] | Yang Y. W., Sun Y. L., Song N., Acc. Chem. Res., 2014, 47(7), 1950—1960 |
| [12] | Tan L. L., Li H., Qiu Y. C., Chen D. X., Wang X., Pan R. Y., Wang Y., Zhang X. A., Wang B., Yang Y. W., Chemical Science,2014, 3, 1640—1644 |
| [13] | Tseng H. R., Vignon S. A., Stoddart J. F., Angew. Chem. Int. Ed., 2003, 115(13), 1529—1533 |
| [14] | Kang S., Vignon S. A., Tseng H. R., Stoddart J. F., Chem. Eur. J., 2004, 10(10), 2555—2564 |
| [15] | Choi J. W., Flood A. H., Steuerman D. W., Nygaard S., Braunschweig A. B., Moonen N. N., Laursen B. W., Luo Y., DeIonno E., Chem. Eur. J., 2006, 12(1), 261—279 |
| [16] | Wu B., Wang T., Feng Y., Zhang Z., Jiang L., Wang C., Nat. Commun., 2015, 6, 6468 |
| [17] | Berná J., Bottari G., Leigh D. A., Pérez E. M., Pure Appl. Chem., 2007, 79(1), 39—54 |
| [18] | Eelkema R., Pollard M. M., Vicario J., Katsonis N., Ramon B. S., Bastiaansen C. W., Broer D. J., Feringa B. L., Nat., 2006, 440(7081), 163 |
| [19] | Barboiu M., Vaughan G., Kyritsakas N., Lehn J. M., Chem. Eur. J., 2003, 9(3), 763—769 |
| [20] | Checińska A., Pollock F. A., Heaney L., Nazir A., J. Chem. Phys., 2015, 142(2), 025102 |
| [21] | Jimenez-Molero M. C., Dietrich-Buchecker C., Sauvage J. P., Chem. Commun., 2003, 14(14), 1613—1616 |
| [22] | Olsen S. T., Elm J., Storm F. E., Gejl A. N., Hansen A. S., Hansen M. H., Nikolajsen J. R., Nielsen M. B., Kjaergaard H. G., Mikkelsen K. V., J. Phys. Chem. A,2015, 119(5), 896—904 |
| [23] | Azov V. A., Beeby A., Cacciarini M., Cheetham A. G., Diederich F., Frei M., Gimzewski J. K., Gramlich V., Hecht B., Jaun B., Adv. Funct. Mater., 2006, 16(2), 147—156 |
| [24] | Sun W. F., Wang D. Y., Chinese J. Org. Chem., 1994, 14, 28 |
| [25] | Seidel D., Lynch V., Sessler J. L., Angew. Chem. Int. Ed., 2002, 41(8), 1422—1425 |
| [26] | Shin J. Y., Furuta H., Yoza K., Igarashi S., Osuka A., J. Am. Chem. Soc., 2001, 123(29), 7190—7191 |
| [27] | Frei M., Marotti F., Diederich F., Chem. Commun., 2004, 12(12), 1362—1363 |
| [28] | Zampolli S., Betti P., Elmi I., Dalcanale E., Chem. Commun., 2007, 27, 2790—2792 |
| [29] | Purse B. W., Butterfield S. M., Ballester P., Shivanyuk A., Rebek J. J., J. Org. Chem., 2008, 73(17), 6480—6488 |
| [30] | Restorp P., Rebek J. J., J. Am. Chem. Soc., 2008, 130(36), 11850—11851 |
| [31] | Wang C., Zhang D., Zhu D., J. Am. Chem. Soc., 2005, 127(47), 16372—16373 |
| [32] | Tang Q., Li H., He M., Hu W., Liu C., Chen K., Wang C., Liu Y., Zhu D., Adv. Mater., 2006, 18(1), 65—68 |
| [33] | Wang C., Zhang D., Zhu D., Langmuir, 2007, 23 (3), 1478—1482 |
| [34] | Gottschalk T., Jarowski P. D., Diederich F., Tetrahedron, 2008, 64(36), 8307—8317 |
| [1] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [2] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [3] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [4] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [5] | WU Zexin, ZHU Yuanjie, WANG Hongzhong, WANG Junan, HE Ying. Methyl-modified Carbazole/Diphenyl Sulfone-based AIE-TADF Blue Emitter and Its OLEDs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220371. |
| [6] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [7] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [8] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [9] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [10] | LI Ran, ZHANG Xudong, MU Lidan, SUN Tong, AI Ganggang, SHA Yelong, ZHANG Yuqi, WANG Jijiang. Preparation and Application of Triplethiophene Derivative Functionalized SiO2 Inverse Opal Photonic Crystal Fluorescent Films [J]. Chem. J. Chinese Universities, 2021, 42(9): 2989. |
| [11] | YUAN Chunling, YAO Xiaotiao, XU Yuanjin, QIN Xiu, SHI Rui, CHENG Shiqi, WANG Yilin. Colorimetry/Ratio Fluorimetry Determination of Glucose with Bifunctional Carbon Dots [J]. Chem. J. Chinese Universities, 2021, 42(8): 2428. |
| [12] | ZHOU Jieqiong, HUANG Yan, ZHANG Zhiling, PANG Daiwen, TIAN Zhiquan. Water-soluble Ag2Te Quantum Dots with Emission in the Second Near-infrared Window [J]. Chem. J. Chinese Universities, 2021, 42(6): 2072. |
| [13] | CHEN Hongda, ZHANG Hua, WANG Zhenxin. Development of Small Animals in vivo Fluorescence-photothermal Dual Mode Imaging System [J]. Chem. J. Chinese Universities, 2021, 42(3): 725. |
| [14] | GONG Yiran, CHEN Changbing, MAO Qiannan, MA Dongliang, LIU Guoquan, YANG Guojian, ZHANG Yumo. Chiroptical Molecular Switches Based on Reversible Electroacid/Electrobase [J]. Chem. J. Chinese Universities, 2021, 42(12): 3651. |
| [15] | CHEN Xiaoyu, LIU Yisheng, HE Mu, SHANGGUAN Ping, HAN Lulu, WANG Jiefei, SHI Bingyang. Recent Advances in Fabricating the Multifunctional Aβ Small Organic Molecule Probes for Theranostic Application [J]. Chem. J. Chinese Universities, 2021, 42(11): 3310. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||