

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (9): 1759.doi: 10.7503/cjcu20150187
• Physical Chemistry • Previous Articles Next Articles
LI Zhaowan, QIAO Zhanping*( ), CHEN Xin, DANG Yuanlin, YANG Qichao
), CHEN Xin, DANG Yuanlin, YANG Qichao
Received:2015-03-11
Online:2015-09-10
Published:2015-08-21
Contact:
QIAO Zhanping
E-mail:nyqiaozp@163.com
Supported by:CLC Number:
TrendMD:
LI Zhaowan, QIAO Zhanping, CHEN Xin, DANG Yuanlin, YANG Qichao. Equilibria for the CsBr-TmBr3-H2O and CsBr-TmBr3-HBr(~13%)-H2O Systems at 298.15 K and Thermodynamic and Fluorescent Properties of New Solid-phase Compound[J]. Chem. J. Chinese Universities, 2015, 36(9): 1759.
| No. | Saturated solution, wb(%) | Wet residue, w(%) | Solid phasec | No. | Saturated solution, wb(%) | Wet residue, w(%) | Solid phasec | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CsBr | TmBr3 | CsBr | TmBr3 | CsBr | TmBr3 | CsBr | TmBr3 | ||||
| 1 | 55.51 | 0 | A | 13 | 13.27 | 56.88 | 40.46 | 41.39 | A+B | ||
| 2 | 48.40 | 6.44 | 94.59 | 0.63 | A | 14 | 12.58 | 57.00 | 24.11 | 51.77 | B |
| 3 | 40.46 | 13.37 | 92.41 | 1.77 | A | 15 | 11.54 | 58.00 | 23.04 | 52.78 | B |
| 4 | 33.45 | 20.09 | 91.82 | 2.49 | A | 16 | 9.90 | 59.66 | 20.91 | 54.28 | B |
| 5 | 29.88 | 24.58 | 90.61 | 3.33 | A | 17 | 8.83 | 60.99 | 14.98 | 59.40 | B+C |
| 6 | 25.87 | 29.73 | 89.52 | 4.18 | A | 18 | 8.70 | 60.75 | 10.29 | 63.33 | B+C |
| 7 | 22.21 | 34.74 | 88.29 | 5.48 | A | 19 | 8.88 | 60.47 | 5.59 | 67.24 | B+C |
| 8 | 18.99 | 39.05 | 90.07 | 4.64 | A | 20 | 6.29 | 62.36 | 2.27 | 69.74 | C |
| 9 | 16.09 | 45.53 | 82.54 | 9.94 | A | 21 | 4.29 | 63.31 | 1.77 | 67.79 | C |
| 10 | 14.86 | 49.22 | 81.76 | 10.57 | A | 22 | 2.75 | 64.29 | 1.42 | 67.83 | C |
| 11 | 14.40 | 51.57 | 82.03 | 10.94 | A | 23 | 0 | 65.67 | C | ||
| 12 | 13.82 | 54.72 | 82.47 | 11.47 | A | ||||||
Table 1 Solubility data(mass fraction, %) for the ternary system CsBr-TmBr3-H2O at (298.15±0.1) K and (0.1±0.005) MPaa
| No. | Saturated solution, wb(%) | Wet residue, w(%) | Solid phasec | No. | Saturated solution, wb(%) | Wet residue, w(%) | Solid phasec | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CsBr | TmBr3 | CsBr | TmBr3 | CsBr | TmBr3 | CsBr | TmBr3 | ||||
| 1 | 55.51 | 0 | A | 13 | 13.27 | 56.88 | 40.46 | 41.39 | A+B | ||
| 2 | 48.40 | 6.44 | 94.59 | 0.63 | A | 14 | 12.58 | 57.00 | 24.11 | 51.77 | B |
| 3 | 40.46 | 13.37 | 92.41 | 1.77 | A | 15 | 11.54 | 58.00 | 23.04 | 52.78 | B |
| 4 | 33.45 | 20.09 | 91.82 | 2.49 | A | 16 | 9.90 | 59.66 | 20.91 | 54.28 | B |
| 5 | 29.88 | 24.58 | 90.61 | 3.33 | A | 17 | 8.83 | 60.99 | 14.98 | 59.40 | B+C |
| 6 | 25.87 | 29.73 | 89.52 | 4.18 | A | 18 | 8.70 | 60.75 | 10.29 | 63.33 | B+C |
| 7 | 22.21 | 34.74 | 88.29 | 5.48 | A | 19 | 8.88 | 60.47 | 5.59 | 67.24 | B+C |
| 8 | 18.99 | 39.05 | 90.07 | 4.64 | A | 20 | 6.29 | 62.36 | 2.27 | 69.74 | C |
| 9 | 16.09 | 45.53 | 82.54 | 9.94 | A | 21 | 4.29 | 63.31 | 1.77 | 67.79 | C |
| 10 | 14.86 | 49.22 | 81.76 | 10.57 | A | 22 | 2.75 | 64.29 | 1.42 | 67.83 | C |
| 11 | 14.40 | 51.57 | 82.03 | 10.94 | A | 23 | 0 | 65.67 | C | ||
| 12 | 13.82 | 54.72 | 82.47 | 11.47 | A | ||||||
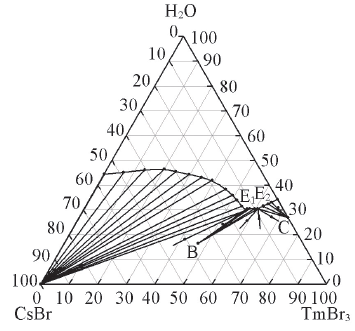
Fig.1 Phase diagram for the ternary system CsBr-TmBr3-H2O at 298.15 KE1 and E2 stand for double saturation point. B and C stand for Cs3Tm2Br9·16H2O and TmBr3·8H2O, respectively.
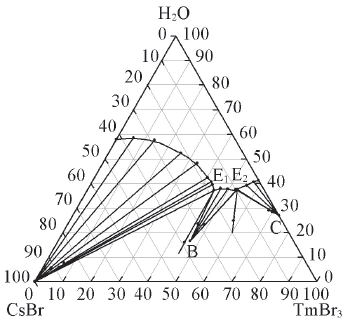
Fig.2 Phase diagram for the quaternary system CsBr-TmBr3-HBr(~13%)-H2O projected on CsBr-TmBr3-H2O at 298.15 KE1 and E2 stand for double saturation point. B and C stand for Cs3Tm2Br9·16H2O and TmBr3·8H2O, respectively.
| No. | Composition of solution, w(%) | Composition of residue, w(%) | Solid phasec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In the tetrahedron | In the trigonal basal faceb | In the tetrahedron | In the trigonal basal face | ||||||||
| HBr | CsBr | TmBr3 | CsBr | TmBr3 | HBr | CsBr | TmBr3 | CsBr | TmBr3 | ||
| 1 | 12.46 | 36.77 | 0.00 | 42.00 | 0.00 | A | |||||
| 2 | 13.81 | 30.76 | 5.02 | 35.69 | 5.83 | 1.83 | 91.28 | 0.69 | 92.98 | 0.71 | A |
| 3 | 13.06 | 24.84 | 11.93 | 28.58 | 13.73 | 1.84 | 91.93 | 1.35 | 93.66 | 1.37 | A |
| 4 | 13.88 | 18.90 | 22.08 | 21.94 | 25.64 | 1.96 | 89.45 | 3.02 | 91.24 | 3.08 | A |
| 5 | 13.45 | 15.88 | 29.02 | 18.34 | 33.53 | 1.60 | 90.15 | 3.23 | 91.61 | 3.28 | A |
| 6 | 13.57 | 15.17 | 34.69 | 17.55 | 40.13 | 2.33 | 86.34 | 5.59 | 88.40 | 5.72 | A |
| 7 | 13.89 | 14.64 | 36.42 | 17.00 | 42.30 | 2.55 | 85.03 | 6.61 | 87.25 | 6.78 | A |
| 8 | 13.76 | 14.77 | 37.42 | 17.13 | 43.39 | 2.56 | 83.54 | 6.53 | 85.73 | 6.70 | A |
| 9 | 13.24 | 15.40 | 39.09 | 17.75 | 45.05 | 6.51 | 36.32 | 42.22 | 38.85 | 45.16 | A+B |
| 10 | 13.05 | 13.11 | 40.82 | 15.08 | 46.95 | 6.63 | 28.25 | 43.11 | 30.26 | 46.18 | B |
| 11 | 13.45 | 11.07 | 42.86 | 12.79 | 49.52 | 6.11 | 28.27 | 43.92 | 30.11 | 46.77 | B |
| 12 | 12.40 | 8.82 | 46.33 | 10.07 | 52.89 | 5.66 | 26.84 | 45.62 | 28.45 | 48.36 | B |
| 13 | 12.72 | 8.25 | 46.23 | 9.45 | 52.97 | 4.26 | 16.33 | 55.57 | 17.06 | 58.04 | B+C |
| 14 | 12.70 | 8.66 | 46.25 | 9.93 | 52.98 | 3.77 | 2.53 | 65.86 | 2.63 | 68.44 | C |
| 15 | 12.52 | 4.59 | 48.61 | 5.25 | 55.57 | 3.08 | 1.53 | 67.96 | 1.58 | 70.12 | C |
| 16 | 12.33 | 1.87 | 50.22 | 2.13 | 57.28 | 2.80 | 1.00 | 68.79 | 1.03 | 70.77 | C |
| 17 | 12.28 | 0.00 | 51.46 | 0.00 | 58.66 | C | |||||
Table 2 Solubility data(mass fraction, %) of the saturated solution of the quaternary system CsBr-TmBr3-HBr(13.09%)-H2O and central projection data on the trigonal basal face CsBr-TmBr3-H2O at (298.15±0.1) K and (0.1±0.005) MPaa
| No. | Composition of solution, w(%) | Composition of residue, w(%) | Solid phasec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In the tetrahedron | In the trigonal basal faceb | In the tetrahedron | In the trigonal basal face | ||||||||
| HBr | CsBr | TmBr3 | CsBr | TmBr3 | HBr | CsBr | TmBr3 | CsBr | TmBr3 | ||
| 1 | 12.46 | 36.77 | 0.00 | 42.00 | 0.00 | A | |||||
| 2 | 13.81 | 30.76 | 5.02 | 35.69 | 5.83 | 1.83 | 91.28 | 0.69 | 92.98 | 0.71 | A |
| 3 | 13.06 | 24.84 | 11.93 | 28.58 | 13.73 | 1.84 | 91.93 | 1.35 | 93.66 | 1.37 | A |
| 4 | 13.88 | 18.90 | 22.08 | 21.94 | 25.64 | 1.96 | 89.45 | 3.02 | 91.24 | 3.08 | A |
| 5 | 13.45 | 15.88 | 29.02 | 18.34 | 33.53 | 1.60 | 90.15 | 3.23 | 91.61 | 3.28 | A |
| 6 | 13.57 | 15.17 | 34.69 | 17.55 | 40.13 | 2.33 | 86.34 | 5.59 | 88.40 | 5.72 | A |
| 7 | 13.89 | 14.64 | 36.42 | 17.00 | 42.30 | 2.55 | 85.03 | 6.61 | 87.25 | 6.78 | A |
| 8 | 13.76 | 14.77 | 37.42 | 17.13 | 43.39 | 2.56 | 83.54 | 6.53 | 85.73 | 6.70 | A |
| 9 | 13.24 | 15.40 | 39.09 | 17.75 | 45.05 | 6.51 | 36.32 | 42.22 | 38.85 | 45.16 | A+B |
| 10 | 13.05 | 13.11 | 40.82 | 15.08 | 46.95 | 6.63 | 28.25 | 43.11 | 30.26 | 46.18 | B |
| 11 | 13.45 | 11.07 | 42.86 | 12.79 | 49.52 | 6.11 | 28.27 | 43.92 | 30.11 | 46.77 | B |
| 12 | 12.40 | 8.82 | 46.33 | 10.07 | 52.89 | 5.66 | 26.84 | 45.62 | 28.45 | 48.36 | B |
| 13 | 12.72 | 8.25 | 46.23 | 9.45 | 52.97 | 4.26 | 16.33 | 55.57 | 17.06 | 58.04 | B+C |
| 14 | 12.70 | 8.66 | 46.25 | 9.93 | 52.98 | 3.77 | 2.53 | 65.86 | 2.63 | 68.44 | C |
| 15 | 12.52 | 4.59 | 48.61 | 5.25 | 55.57 | 3.08 | 1.53 | 67.96 | 1.58 | 70.12 | C |
| 16 | 12.33 | 1.87 | 50.22 | 2.13 | 57.28 | 2.80 | 1.00 | 68.79 | 1.03 | 70.77 | C |
| 17 | 12.28 | 0.00 | 51.46 | 0.00 | 58.66 | C | |||||
| No. | m/mg | Qs/mJ | Δsol |
|---|---|---|---|
| 1 | 64.80 | -416.9 | -11.22 |
| 2 | 65.72 | -449.7 | -11.93 |
| 3 | 65.27 | -445.5 | -11.90 |
| 4 | 67.88 | -470.1 | -12.07 |
| 5 | 63.79 | -404.3 | -11.05 |
| Meanb | -(11.63±0.46) | ||
Table 3 Molar enthalpy of solution of Cs3Tm2Br9·16H2O in deionized water at (298.15±0.01) Ka
| No. | m/mg | Qs/mJ | Δsol |
|---|---|---|---|
| 1 | 64.80 | -416.9 | -11.22 |
| 2 | 65.72 | -449.7 | -11.93 |
| 3 | 65.27 | -445.5 | -11.90 |
| 4 | 67.88 | -470.1 | -12.07 |
| 5 | 63.79 | -404.3 | -11.05 |
| Meanb | -(11.63±0.46) | ||
| [1] | Li Y. X., Xue M., Guo L. J., Huang L., Chen S. R., Qiu S. L., Chem. Res. Chinese Universities, 2013, 29(2), 196—200 |
| [2] | Xu Z. H., Gao Y., Ge X., Sun Y. G., Chem. Res. Chinese Universities, 2013, 29(1), 1—5 |
| [3] | Güdel H. U., Furrer A., Blank H., Inorg. Chem., 1990, 29, 4081—4084 |
| [4] | Aebersold M. A., Güdel H. U., Furrer A., Blank H., Inorg. Chem., 1994, 33, 1133—1138 |
| [5] | Hehlem M. P., Krämer K., Güdel H. U., Phys. Rev. B: Condens. Matter, 1994, 49, 12475—12483 |
| [6] | Hehlen M. P., Güdel H. U., Quagliano J. R., J. Chem. Phys., 1994, 101, 10303—10312 |
| [7] | Leszek R., Marcelle G. E., J. Therm. Anal. Calorim., 1999, 56, 355—363 |
| [8] | Leszek R., Ewa I. S., Marcelle G. E., J. Therm. Anal. Calorim., 2009, 97, 1015—1021 |
| [9] | Leszek R., Ewa I. S., Berkani M., Marcelle G. E., Z. Naturforsch., 2010, 65a, 859—864 |
| [10] | Leszek R., Ida C., Jan K., Marcelle G. E., Calphad, 2012, 37, 108—115 |
| [11] | Chojnackaa I., Leszek R., Berkanib M., Marcelle G. E., J. Alloy Compd., 2014, 582, 505—510 |
| [12] | Li Y. L., Chen C., Qiao Z. P., Li Z. W., J. Chem. Eng. Data, 2014, 59, 3125—3129 |
| [13] | Wang H., Ran X. Q., Chen P. H., Chem. J. Chinese Universities, 1995, 16(11), 1660—1663 |
| (王惠, 冉新权, 陈佩珩. 高等学校化学学报, 1995, 16(11), 1660—1663) | |
| [14] | Wang H., Ran X. Q., Chen P. H., Chin. Sci. Bull., 1996, 41(11), 910—915 |
| [15] | Wang H., Ran X. Q., Chen P. H., Chem. J. Chinese Universities, 1997, 18(9), 1420—1424 |
| (王惠, 冉新权, 陈佩珩. 高等学校化学学报, 1997, 18(9), 1420—1424) | |
| [16] | Wang H., Ran X. Q., Chen P. H., Acta Chim. Sinica, 1997, 13(2), 169—172 |
| (王惠, 冉新权, 陈佩珩. 物理化学学报, 1997, 13(2), 169—172) | |
| [17] | Wang H., Ran X. Q., Chen P. H., Chem. J. Chinese Universities, 1996, 17(11), 1670—1672 |
| (王惠, 冉新权, 陈佩珩. 高等学校化学学报, 1996, 17(11), 1670—1672) | |
| [18] | Qiao Z. P., Chen J. G., Zhuo L. H., Zhang S. S., Russ. J. Inorg. Chem., 2008, 53, 450—454 |
| [19] | Qiao Z. P., Li Y. L., Dang Y. L., Xie H. Q., J. Chem. Thermodynamics, 2014, 68, 275—280 |
| [20] | Chen Y.S., Analysis of Physical Chemistry, Higher Education Press, Beijing, 1988, 285—286 |
| (陈运生. 物理化学分析, 北京: 高等教育出版社, 1988, 285—286) | |
| [21] | Ji M., Liu M. Y., Gao S. L., J. Instrum. Sci. Technol., 2001, 29, 53—57 |
| [22] | Rychly R., Pekárek V., J. Chem. Thermodynamics, 1977, 9, 391—396 |
| [23] | Qiao Z. P., Zhuo L. H., Zhang S. S., Wang H., Chinese J. Inorg. Chem., 2006, 22(8), 1545—1549 |
| (乔占平, 卓立宏, 张书申, 王惠. 无机化学学报, 2006, 22(8), 1545—1549) | |
| [24] | Wagman D.D., Evans W. H., Parker V. B., Schumm R. H., Halow I., Bailey S. M., Chumey K. L., Nuttal R. L., The NBS Tables of Chemical Thermodynamic Properties, Translated by Liu T. H., Zhao M. Y., China Standards Press, Beijing, 1998, 50—367 |
| (Wagman D.D., Evans W. H., Parker V. B., Schumm R. H., Halow I., Bailey S. M., Chumey K. L., Nuttal R. L. 刘天和, 赵梦月[译], NBS化学热力学性质表, 北京: 中国标准出版社, 1998, 50—367) | |
| [25] | Cox J.D., Wagman D. D., Medvedev V. A., CODATA Key Values for Thermodynamics, Hemisphere,New York, 1989, 20—21 |
| [1] | WANG Xingfan, ZHOU Huan, ZHOU Kuo, JIN Fenli, YANG Junfang. Comprehensive Thermodynamic Model and Polythermal Phase Diagram Prediction of Nitrate Type Brine Systems [J]. Chem. J. Chinese Universities, 2021, 42(10): 3175. |
| [2] | WANG Dongmei,LIU Zihua,LI Guanghua,LIU Yunling,LI Chunxia. Synthesis, Structure and Fluorescent Property of Indium-based Bimetallic Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1886. |
| [3] | LIU Shujing, LI Jiangtao, GU Fang, WANG Haijun. Phase Structure of Aa Type of Patchy Colloids† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1888. |
| [4] | LIU Xuewu,CHEN Shuhua,ZHAN Shiping. Experimental Study and Theoretical Phase Diagram Calculation for Polystyrene Membranes Prepared by Supercritical CO2-induced Phase Inversion† [J]. Chem. J. Chinese Universities, 2016, 37(8): 1573. |
| [5] | DING Yanli, HU Shengliang, CHANG Qing. Preparation and Characterization of Composites of Amine-functionalized Carbon Dots and Zinc Phthalocyaine† [J]. Chem. J. Chinese Universities, 2015, 36(4): 619. |
| [6] | HU Biao, ZHOU Peng, PAN Chengling, DU Yong, LIU Shuhong, LI Yiwei, HAN Jiangjun. Theoretical Study on the Binary Phase Diagrams of the Zr-X (X=Li, Na, K, Sc, Hf) Systems† [J]. Chem. J. Chinese Universities, 2015, 36(10): 1939. |
| [7] | YIN Huiqin, WANG Kun, LIU Wenguan, XIE Leidong, HAN Han, WANG Wenfeng. Thermodynamic Modeling of the LiF-CrF3 Binary System† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2668. |
| [8] | ZHU Yan, YAO Wei-Qin, CHEN Shao-Jie, TIAN Long-Zhao, YUAN Shuai. Phase Diagram and Temperature Influence of SDS/n-pentanol-cyclohexane-water Pseudo Ternary Systems [J]. Chem. J. Chinese Universities, 2013, 34(5): 1254. |
| [9] | GAO Zhen-Fei, DI Ming-Zhe, DI You-Ying. Synthesis, Structure and Thermochemistry of Pyridine-2,6-dicarboxylic Acid Lithium Hydrogen [J]. Chem. J. Chinese Universities, 2013, 34(5): 1208. |
| [10] | ZHONG Wen-Wen, DI Ming-Zhe, DI You-Ying. Synthesis, Structure Characterization and Thermochemistry of Pyridine-2,6-dicarboxylic Acid Potassium Hydrogen [J]. Chem. J. Chinese Universities, 2012, 33(09): 2074. |
| [11] | ZHANG Zhi-Wei, DING Wei, LI Zhong, DONG Zhi-Long, YU Tao, QU Guang-Miao, WANG Hui-Min, LUO Shi-Qiong. Properties of Microemulsion Formed by Different Alkyl Aryl Sulfonates [J]. Chem. J. Chinese Universities, 2012, 33(02): 395. |
| [12] | HUANG Xue-Li*, WANG Xue-Feng, CHEN Li-Juan. Liquid-Solid Equilibrium of Na+, K+, Mg2+//Cl-, SO2-4, NO-3, H2O System at 25 ℃ [J]. Chem. J. Chinese Universities, 2011, 32(6): 1378. |
| [13] | ZHU Yan* , WEI Ning-Bo, YUAN Shuai, XIAO Bing. Studies on Phase Diagram and Conductivity of SDS/n-Pentanol-xylene-water(Salt Solution) Pseudo Ternary Systems and Preparation of Nano ZnO [J]. Chem. J. Chinese Universities, 2010, 31(7): 1405. |
| [14] | LIU Yu-Pu, DI You-Ying*, HE Dong-Hua, KONG Yu-Xia, YANG Wei-Wei, DAN Wen-Yan. Low-temperature Heat Capacities and Thermochemistry Properties of Ethylene Diamine Dihydrochloride [J]. Chem. J. Chinese Universities, 2010, 31(6): 1227. |
| [15] | GAO Gui-Bo, QIAN Chun-Xiang*. Phase Transition Capability of the Binary System Capric Acid-Myristic Acid [J]. Chem. J. Chinese Universities, 2009, 30(8): 1658. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||