

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (3): 411.doi: 10.7503/cjcu20140986
• Articles: Inorganic Chemistry • Previous Articles Next Articles
HUANG Jian1, LIU Zhigang1, FAN Ruiqing1,*( ), DONG Yuwei1, ZHANG Huijie1, WANG Yulei2, YANG Yulin1,*(
), DONG Yuwei1, ZHANG Huijie1, WANG Yulei2, YANG Yulin1,*( )
)
Received:2014-11-17
Online:2015-03-10
Published:2015-02-04
Contact:
FAN Ruiqing,YANG Yulin
E-mail:fanruiqing@hit.edu.cn;ylyang@hit.edu.cn
Supported by:CLC Number:
TrendMD:
HUANG Jian, LIU Zhigang, FAN Ruiqing, DONG Yuwei, ZHANG Huijie, WANG Yulei, YANG Yulin. Synthesis, Characterization and Blue-emission of Cd(Ⅱ)Coordination Polymers with μ-Chlorine-Bridging One-dimensional Chain Structures†[J]. Chem. J. Chinese Universities, 2015, 36(3): 411.
| Complex | 1 | 2 |
|---|---|---|
| Formula | C14H14CdCl2N2 | C7H7CdCl2NO |
| Molecular weight | 393.58 | 304.45 |
| Crystal system | Triclinic | Triclinic |
| Space group | P | P |
| a/nm | 0.7493(1) | 0.7264(2) |
| b/nm | 0.9586(1) | 0.8328(2) |
| c/nm | 1.0492(1) | 0.9328(1) |
| α/(°) | 91.858(2) | 109.49(2) |
| β/(°) | 102.787(2) | 94.30(1) |
| γ/(°) | 92.890(2) | 114.36(3) |
| Volume/nm3 | 0.7333(1) | 0.4695(1) |
| Z | 2 | 2 |
| T/K | 298 | 298 |
| Dc/(g·cm-3) | 1.783 | 2.154 |
| μ/mm-1 | 1.840 | 2.843 |
| F(000) | 388 | 292 |
| θrange | 1.99° to 25.00° | 3.73° to 27.56° |
| Number of reflections collected | 5186(Rint=0.0265) | 2763(Rint=0.0235) |
| Number of independent reflections | 2560 | 2134 |
| Number of data/restraints/parameters | 2560/0/172 | 2134/0/110 |
| GOF | 1.081 | 0.997 |
| R*[I >2σ(I)] | R1=0.0327, wR2=0.0840 | R1=0.0313, wR2=0.0709 |
Table 1 Crystal data and refinement parameters of complexes 1 and 2
| Complex | 1 | 2 |
|---|---|---|
| Formula | C14H14CdCl2N2 | C7H7CdCl2NO |
| Molecular weight | 393.58 | 304.45 |
| Crystal system | Triclinic | Triclinic |
| Space group | P | P |
| a/nm | 0.7493(1) | 0.7264(2) |
| b/nm | 0.9586(1) | 0.8328(2) |
| c/nm | 1.0492(1) | 0.9328(1) |
| α/(°) | 91.858(2) | 109.49(2) |
| β/(°) | 102.787(2) | 94.30(1) |
| γ/(°) | 92.890(2) | 114.36(3) |
| Volume/nm3 | 0.7333(1) | 0.4695(1) |
| Z | 2 | 2 |
| T/K | 298 | 298 |
| Dc/(g·cm-3) | 1.783 | 2.154 |
| μ/mm-1 | 1.840 | 2.843 |
| F(000) | 388 | 292 |
| θrange | 1.99° to 25.00° | 3.73° to 27.56° |
| Number of reflections collected | 5186(Rint=0.0265) | 2763(Rint=0.0235) |
| Number of independent reflections | 2560 | 2134 |
| Number of data/restraints/parameters | 2560/0/172 | 2134/0/110 |
| GOF | 1.081 | 0.997 |
| R*[I >2σ(I)] | R1=0.0327, wR2=0.0840 | R1=0.0313, wR2=0.0709 |
| Cd1—N1 | 0.2347(4) | Cd1—Cl1 | 0.2667(1) | Cd1—Cl2 | 0.2500(1) |
|---|---|---|---|---|---|
| Cd1— N2 | 0.2404(3) | Cd1—Cl1#1 | 0.2606(1) | Cd1—Cl2#2 | 0.2866(1) |
| N1—Cd1—N2 | 69.40(3) | Cl2—Cd1—Cl1#1 | 101.14(4) | N1—Cd1—Cl2#2 | 78.86(1) |
| N1—Cd1—Cl2 | 157.42(1) | N1—Cd1—Cl1 | 96.92(1) | N2—Cd1—Cl2#2 | 90.99(9) |
| N2—Cd1—Cl2 | 102.89(9) | N2—Cd1—Cl1 | 84.73(9) | Cl2—Cd1—Cl2#2 | 80.13(4) |
| N1—Cd1—Cl1#1 | 90.67(9) | Cl2—Cd1—Cl1 | 103.58(4) | Cl1#1—Cd1—Cl2#2 | 100.28(4) |
| N2—Cd1—Cl1#1 | 154.87(9) | Cl1#1—Cd1—Cl1 | 82.62(4) | Cl1—Cd1—Cl2#2 | 174.85(4) |
Table 2 Selected bond distances(nm) and bond angles(°) of complexs 1*
| Cd1—N1 | 0.2347(4) | Cd1—Cl1 | 0.2667(1) | Cd1—Cl2 | 0.2500(1) |
|---|---|---|---|---|---|
| Cd1— N2 | 0.2404(3) | Cd1—Cl1#1 | 0.2606(1) | Cd1—Cl2#2 | 0.2866(1) |
| N1—Cd1—N2 | 69.40(3) | Cl2—Cd1—Cl1#1 | 101.14(4) | N1—Cd1—Cl2#2 | 78.86(1) |
| N1—Cd1—Cl2 | 157.42(1) | N1—Cd1—Cl1 | 96.92(1) | N2—Cd1—Cl2#2 | 90.99(9) |
| N2—Cd1—Cl2 | 102.89(9) | N2—Cd1—Cl1 | 84.73(9) | Cl2—Cd1—Cl2#2 | 80.13(4) |
| N1—Cd1—Cl1#1 | 90.67(9) | Cl2—Cd1—Cl1 | 103.58(4) | Cl1#1—Cd1—Cl2#2 | 100.28(4) |
| N2—Cd1—Cl1#1 | 154.87(9) | Cl1#1—Cd1—Cl1 | 82.62(4) | Cl1—Cd1—Cl2#2 | 174.85(4) |
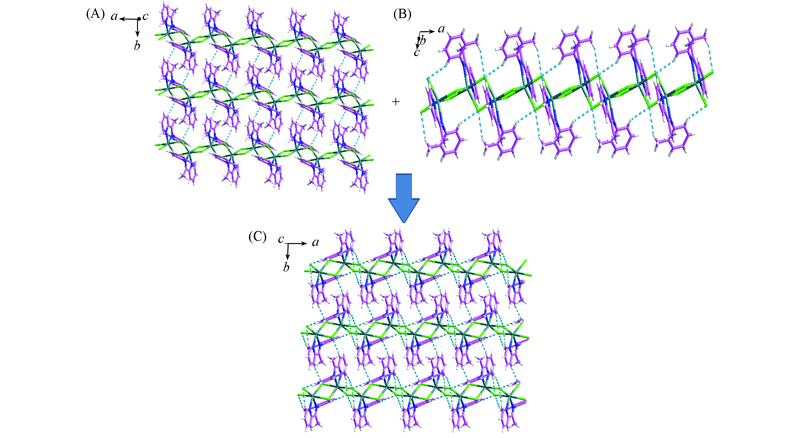
Fig.2 Intermolecular(A) and intramolecular(B) hydrogen bonds in complex 1 and supramolecular structure of complex 1(C) Hydrogen bonds are illustrates by dashed lines.
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| C5—H5A…Cl1A | 0.093 | 0.2795 | 0.3468 | 130 |
| C2—H2A…Cl2 | 0.093 | 0.2699 | 0.3575 | 157 |
| Cl3—H13A…Cl1 | 0.093 | 0.2757 | 0.3532 | 141 |
| C14—H14B…Cl1 | 0.096 | 0.2808 | 0.3704 | 155 |
Table 3 Hydrogen bond lengths and bond angles of complex 1
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| C5—H5A…Cl1A | 0.093 | 0.2795 | 0.3468 | 130 |
| C2—H2A…Cl2 | 0.093 | 0.2699 | 0.3575 | 157 |
| Cl3—H13A…Cl1 | 0.093 | 0.2757 | 0.3532 | 141 |
| C14—H14B…Cl1 | 0.096 | 0.2808 | 0.3704 | 155 |
| Cd1—N1 | 0.2340(3) | Cd1—Cl1 | 0.2697(1) | Cd1—Cl2 | 0.2504(1) |
|---|---|---|---|---|---|
| Cd1—O1 | 0.2432(3) | Cd1—Cl1#1 | 0.2535(1) | Cd1—Cl2#2 | 0.2729(1) |
| N1—Cd1—O1 | 68.09(1) | Cl2—Cd1—Cl1#1 | 108.17(4) | N1—Cd1—Cl2#2 | 83.62(7) |
| N1—Cd1—Cl2 | 155.43(7) | N1—Cd1—Cl1 | 91.62(7) | O1—Cd1—Cl2#2 | 94.31(7) |
| O1—Cd1—Cl2 | 91.51(7) | O1—Cd1—Cl1 | 82.50(7) | Cl2—Cd1—Cl2#2 | 84.48(4) |
| N1—Cd1—Cl1#1 | 94.67(8) | Cl2—Cd1—Cl1 | 99.39(4) | Cl1#1—Cd1—Cl2#2 | 97.59(4) |
| O1—Cd1—Cl1#1 | 157.82(7) | Cl1#1—Cd1—Cl1 | 84.23(4) | Cl1—Cd1—Cl2#2 | 175.01(3) |
Table 4 Selected bond distances(nm) and bond angles(°) of complexs 2*
| Cd1—N1 | 0.2340(3) | Cd1—Cl1 | 0.2697(1) | Cd1—Cl2 | 0.2504(1) |
|---|---|---|---|---|---|
| Cd1—O1 | 0.2432(3) | Cd1—Cl1#1 | 0.2535(1) | Cd1—Cl2#2 | 0.2729(1) |
| N1—Cd1—O1 | 68.09(1) | Cl2—Cd1—Cl1#1 | 108.17(4) | N1—Cd1—Cl2#2 | 83.62(7) |
| N1—Cd1—Cl2 | 155.43(7) | N1—Cd1—Cl1 | 91.62(7) | O1—Cd1—Cl2#2 | 94.31(7) |
| O1—Cd1—Cl2 | 91.51(7) | O1—Cd1—Cl1 | 82.50(7) | Cl2—Cd1—Cl2#2 | 84.48(4) |
| N1—Cd1—Cl1#1 | 94.67(8) | Cl2—Cd1—Cl1 | 99.39(4) | Cl1#1—Cd1—Cl2#2 | 97.59(4) |
| O1—Cd1—Cl1#1 | 157.82(7) | Cl1#1—Cd1—Cl1 | 84.23(4) | Cl1—Cd1—Cl2#2 | 175.01(3) |
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| C7—H7A…Cl1A | 0.096 | 0.2758 | 0.3494 | 134 |
| C3—H3A…Cl1 | 0.093 | 0.2785 | 0.3570 | 142 |
Table 5 Hydrogen bond lengths and bond angles of complex 2
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| C7—H7A…Cl1A | 0.096 | 0.2758 | 0.3494 | 134 |
| C3—H3A…Cl1 | 0.093 | 0.2785 | 0.3570 | 142 |
| Medium | Complex 1 | Complex 2 | ||
|---|---|---|---|---|
| Emission, λmax/nm | Decay lifetime, τ/μs | Emission, λmax/nm | Decay lifetime, τ/μs | |
| CH2Cl2 | 390 | 38.10 | 400 | 23.83 |
| CH3CN | 410 | 60.20 | 425 | 37.50 |
| CH3OH | 420 | 38.10 | 433 | 19.83 |
| Solid | 440 | 20.20 | 473 | 19.08 |
Table 6 Photoluminescent data for complexes 1 and 2
| Medium | Complex 1 | Complex 2 | ||
|---|---|---|---|---|
| Emission, λmax/nm | Decay lifetime, τ/μs | Emission, λmax/nm | Decay lifetime, τ/μs | |
| CH2Cl2 | 390 | 38.10 | 400 | 23.83 |
| CH3CN | 410 | 60.20 | 425 | 37.50 |
| CH3OH | 420 | 38.10 | 433 | 19.83 |
| Solid | 440 | 20.20 | 473 | 19.08 |
| [1] | Tang C. W., Vaslyke S. A., Appl. Phys. Lett., 1987, 51(12), 913—915 |
| [2] | Frath D., Massue J., Ulrich G., Ziessel R., Angew. Chem. Int. Ed., 2014, 53, 2290—2310 |
| [3] | Martin D., Rouffet M., Cohen S. M., Inorg. Chem., 2010, 49, 10226—10228 |
| [4] | Zhao Q., Li R. F., Xing S. K., Liu X. M., Hu T. L., Bu X. H., Inorg. Chem., 2011, 50, 10041—10046 |
| [5] | Barbieri A., Accosi G., Armaroli N., Chem. Commun., 2008, 19, 2185—2193 |
| [6] | Tang Y. W., Huo Y. P., Hu S., Zhang K., Zhao F. H., Ouyang X. H., Chem. J. Chinese Universities,2014, 35(1), 48—53 |
| (汤胤旻, 霍延平, 胡升, 张焜, 赵丰华, 欧阳新华. >高等学校化学学报, 2014, 35(1), 48—53) | |
| [7] | Liuzzo V., Oberhauser W., Pucci A., Inorg. Chem. Commun., 2010, 13, 686—688 |
| [8] | Dietrich B. L., Egbert J., Morris A. M., Wicholas M., Inorg. Chem., 2005, 44, 6476—6481 |
| [9] | Liu H. Y., Wu H., Ma J. F., Liu Y. Y., Liu B., Yang J., Cryst. Growth Des., 2010, 10, 4795—4805 |
| [10] | Marinescu G., Marin G., Madalan A.M., Vezeanu A., Tiseanu C., Andruh M., Cryst. Growth Des., 2010, 10, 2096—2103 |
| [11] | Su Q., Gao W., Wu Q. L., Ye L., Li G. H., Mu Y., Eur. J. Inorg. Chem., 2007, 26, 4168—4175 |
| [12] | Li G. B., Liu J. M., Cai Y. P., Su C. Y., Cryst. Growth Des., 2011, 11, 2763—2772 |
| [13] | Hatten X. D., Asil D., Friend R. H., Nitschke J. R., J. Am. Chem. Soc., 2012, 134, 19170—19178 |
| [14] | Li K. B., Zhang H. L., Zhu B., He X. P., Xie J., Chen G. R., Dye Pigment,2014, 102, 273—277 |
| [15] | Yang X. P., Schipper D., Jones R. A., Lytwak L. A., Holliday B. J., Huang S. M., J. Am. Chem. Soc., 2013, 135, 8468—8471 |
| [16] | Yang X. P., Lam D., Chan C., Stanley J. M., Jones R. A., Holliday B. J., Wong W. K., Dalton Trans., 2011, 40, 9795—9801 |
| [17] | Aandruh M., Chem. Commun., 2011, 47, 3025—3042 |
| [18] | Cucos P., Tuna F., Sorace L., Maxim I., Maim C., Shova S., Ghorghe R., Caneschi A., Hillebrand M., Andruh M., Inorg. Chem., 2014, 53, 7738—7747 |
| [19] | So H. S., Rao B. A., Hwang J., Yesudas K., Son Y. A., Sens. Actuator B: Chem., 2014, 202, 779—787 |
| [20] | Wang K., Zhao F. C., Wang C. G., Chen S. Y., Chen D., Zhang H. Y., Liu Y., Ma D. G., Wang Y., Adv. Funct. Mater., 2013, 23, 2672—2680 |
| [21] | Su Z. M., Cheng H., Gao H. Z., Sun S. L., Chu B., Wang R. S., Wang Y., Chem. J. Chinese Universities,2000, 21(9), 1416—1421 |
| (苏忠民, 程红, 高洪泽, 孙世玲, 初蓓, 王荣顺, 王悦. 高等学校化学学报, 2000, 21(9), 1416—1421) | |
| [22] | Mukberjee S., Thilagar P., Proc. Natl. Acad. Sci., India Sect. A Phys. Sci., 2014, 84(2), 131—149 |
| [23] | Su Q., Gao W., Wu Q.L., Ye L., Li G. H., Mu Y.,Eur. J. Inorg. Chem., 2007, 4168—4175 |
| [24] | Baul T. S. B., Kundu S., Ng S. W., Guchhait N., Tiekink E. R. T., J. Coord. Chem., 2014, 67(1), 96—119 |
| [25] | Escuer A., Vicente R., Ribas J., Inorg. Chem., 1995, 34(7), 1793—1798 |
| [26] | Zhu L. N., Xu N., Zhang W., Liao D. Z., Yoshimura K., Mibu K., Jiang Z. H., Yan S. P., Cheng P., Inorg. Chem., 2007, 46(4), 1297—1304 |
| [27] | Maiti M., Sadhukhan D., Thakurta S., Roy S., Pilet G., Butcher R. J., Nonat A., Charbonneire L. J., Mitra S., Inorg. Chem., 2012, 51(22), 12176—12187 |
| [28] | Sheldrick G.M., SHELXTL NT Crystal Structure Analysis Package, Version 5.10, Bruker Axs, Analytical X-ray System, Madison, WI, 1999 |
| [29] | Wei G., Shen Y. F., Li Y. R., Huang X. C., Inorg. Chem., 2010, 49, 9191—9199 |
| [30] | Duan L. K., Ding Y. N., Meng X. R., Li W. Q., Hou H. W., Fan Y. T., J. Mol. Struct., 2010, 975, 53—58 |
| [31] | Das K., Konar S., Jana A., Barik A. K., Roy S., Kar S. K., J. Mol. Struct., 2013, 1036, 392—401 |
| [32] | Jin Y., Yu C. H., Wang W. X., Li S. C., Zhang W., Inorg. Chim. Acta,2014, 413, 97—101 |
| [33] | Liu Z. Z., Gao W., Zhang J. S., Cui D. M., Wu Q. L., Mu Y., Organometallics,2010, 29, 5783—5790 |
| [1] | XU Dandan, ZOU Xiucheng, LUO Jing, LIU Ren. Synthesis and Characterization of Phenothiazine-based Schiff Bases as Visible Light Photoinitiators [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210857. |
| [2] | CAO Cheng,CAO Tian,YAN Penji,WANG Qingyun,YUE Guoren,JI Xiangdong. High Selectivity of Enol-keto Tautomers Imine Derivative for the Recognition of Co2+† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1544. |
| [3] | LI Cheng,SONG Jixue,LIU Tingting,LI Yan,LIU Bingnan,WANG Liang,XIAO Shan,LI Lin,GENG Xuhui,WANG Jihui. Detection of Egg White Lysozyme Oligomers Based on Fluorescence Lifetime of ThT† [J]. Chem. J. Chinese Universities, 2019, 40(1): 90. |
| [4] | SUN Jiayin, LU Ke, LIU Guangzhi, LIU Lu, PAN Wenrong, ZHANG Sijing, QIN Wei, WU Genhua. Syntheses, Crystal Structures and Luminescent Properties of Two Magnesium(Ⅱ) Supramolecular Architectures Based on Hydrogen Bonds† [J]. Chem. J. Chinese Universities, 2017, 38(5): 729. |
| [5] | XIONG Xingquan, JIANG Yunbing, XIAO Shangyun, SHI Lin, SONG Sida. Highly Efficient Synthesis of 1,2,3-Triazoles Catalyzed by Schiff Base Functionalized Chitosan-CuBr Catalyst† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1863. |
| [6] | WANG Zijian, SUN Xiaohong, LIU Yuanfa, CHEN Bang, SHEN Shengqiang, JIN Ruyi, MA Haixia. Synthesis and Biological Activities of 1,2,4-Triazole Schiff Bases Containing Pyrazole Rings† [J]. Chem. J. Chinese Universities, 2015, 36(7): 1315. |
| [7] | QU Zhi, LI Xiang, PANG Xuan, DUAN Ranlong, GAO Bo, CHEN Xuesi. Aluminum Schiff Base Catalyst for Ring-opening Polymerization of ε-Caprolactone† [J]. Chem. J. Chinese Universities, 2014, 35(4): 869. |
| [8] | TANG Yinmin, HUO Yanping, HU Sheng, ZHANG Kun, ZHAO Fenghua, OUYANG Xinhua. Synthesis and Photoelectricity of Novel Zn Metal Complex Based on 8-Hydroxyquinoline with Thiophene Group† [J]. Chem. J. Chinese Universities, 2014, 35(1): 48. |
| [9] | ZHANG Shu-Yuan, JIANG Ping-Ping, LENG Yan, XU Yu-Cheng, MO Guan-Tian, BIAN Gang. Synthesis of cis-Dioxomolybdenum(Ⅵ)-tridentate Schiff Base Complexes and Its Catalytic Activity on the Epoxidation of Soybean Oil [J]. Chem. J. Chinese Universities, 2013, 34(7): 1703. |
| [10] | ZHANG Ling-Yun, YANG Yu-Lin, FAN Rui-Qing, ZHANG Yan-Jiao, WANG Ping, LI Liang. Performances of Dye-sensitized Solar Cell Based on ZnO Photoanode Sensitized with Cadmium Complexes Cd(phen)2(NO3)(NO2) [J]. Chem. J. Chinese Universities, 2013, 34(6): 1470. |
| [11] | QIU Zhao-Lai, LI Wen-Hong, ZHU Hai-Fei, LIU Qian, LI Yuan. Synthesis, Crystal Structure and Antifungal Activities of 3-(CH2)nCO2C2H5-1,5-Benzothiazepines [J]. Chem. J. Chinese Universities, 2013, 34(3): 579. |
| [12] | SUN Ying, REN Ai-Min*, LI Zuo-Sheng, MIN Chun-Gang, REN Xue-Feng, FENG Ji-Kang. Theoretical Investigation of the Reaction Mechanism of Cypridina Luciferin Analogues [J]. Chem. J. Chinese Universities, 2011, 32(11): 2586. |
| [13] | YIN De-Fei, CHENG Hong-Bo, HUO Xiao-Lian, LI Hai-Ning, PANG Mei-Li*. Synthesis and Properties of Novel Photochromic Dyads Based on Spiropyrans and Schiff Base [J]. Chem. J. Chinese Universities, 2011, 32(10): 2301. |
| [14] | LI Xiao-Juan, SUN Shi-Ling, LIU Yan, ZHAO Hai-Bo, LIU Chun-Guang, QIU Yong-Qing*. DFT Studies on Electronic Spectra and Second\|order Nonlinear Optical Properties of Schiff Base Ligands Containing Ferrocene and Their Ni(Ⅱ) Complexes [J]. Chem. J. Chinese Universities, 2011, 32(1): 155. |
| [15] | YANG Su-Hua, PANG Mei-Li*, GUO Xin-Fu, LI Jing-Jing, HAN Jie, MENG Ji-Ben. Synthesis and Properties of Novel Photochromic Dyads Based on Spirooxazine and Schiff Base [J]. Chem. J. Chinese Universities, 2010, 31(5): 976. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||