

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (4): 869.doi: 10.7503/cjcu20130959
• Polymer Chemistry • Previous Articles Next Articles
QU Zhi1,2, LI Xiang1, PANG Xuan1, DUAN Ranlong1, GAO Bo1, CHEN Xuesi1,*( )
)
Received:2013-09-29
Online:2014-04-10
Published:2014-02-27
Contact:
CHEN Xuesi
E-mail:xschen@ciac.ac.cn
Supported by:CLC Number:
TrendMD:
QU Zhi, LI Xiang, PANG Xuan, DUAN Ranlong, GAO Bo, CHEN Xuesi. Aluminum Schiff Base Catalyst for Ring-opening Polymerization of ε-Caprolactone†[J]. Chem. J. Chinese Universities, 2014, 35(4): 869.
| Complex | Temperature/℃ | Time/h | Conv.(1H NMR)(%) | 10-3 Mn(GPC) | PDI(GPC) |
|---|---|---|---|---|---|
| 1a | 40 | 20 | 99 | 18.4 | 1.23 |
| 1a | 60 | 10 | 99 | 23.4 | 1.35 |
| 1a | 80 | 5 | 99 | 22.6 | 1.47 |
| 2a | 40 | 20 | 99 | 21.5 | 1.19 |
| 2a | 60 | 10 | 99 | 21.7 | 1.31 |
| 2a | 80 | 5 | 99 | 24.8 | 1.39 |
| 3a | 40 | 20 | 99 | 22.6 | 1.25 |
| 3a | 60 | 10 | 100 | 23.4 | 1.28 |
| 3a | 80 | 5 | 100 | 25.7 | 1.43 |
Table 1 Polymerization data of ε-CL using complexes 1a—3a*
| Complex | Temperature/℃ | Time/h | Conv.(1H NMR)(%) | 10-3 Mn(GPC) | PDI(GPC) |
|---|---|---|---|---|---|
| 1a | 40 | 20 | 99 | 18.4 | 1.23 |
| 1a | 60 | 10 | 99 | 23.4 | 1.35 |
| 1a | 80 | 5 | 99 | 22.6 | 1.47 |
| 2a | 40 | 20 | 99 | 21.5 | 1.19 |
| 2a | 60 | 10 | 99 | 21.7 | 1.31 |
| 2a | 80 | 5 | 99 | 24.8 | 1.39 |
| 3a | 40 | 20 | 99 | 22.6 | 1.25 |
| 3a | 60 | 10 | 100 | 23.4 | 1.28 |
| 3a | 80 | 5 | 100 | 25.7 | 1.43 |
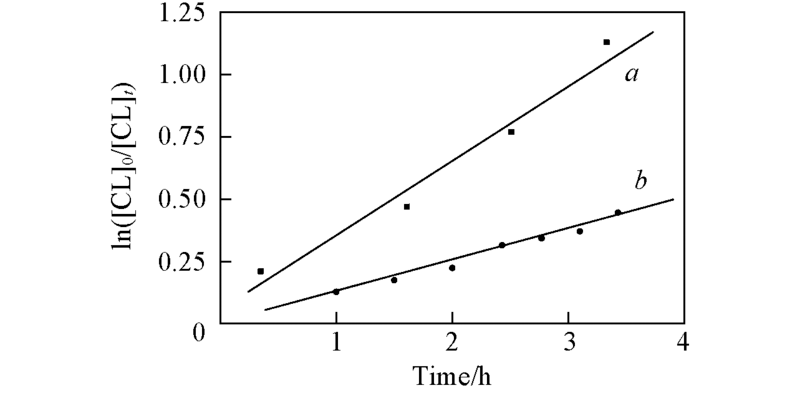
Fig.2 First-order kinetics plots for the ROP of ε-CL with 1a/2-propanol as catalyst/initiator in toluene with 60 ℃ and [CL]0=0.5 mol/L for [CL]/[Cat]=100:1(a) and 200:1(b)
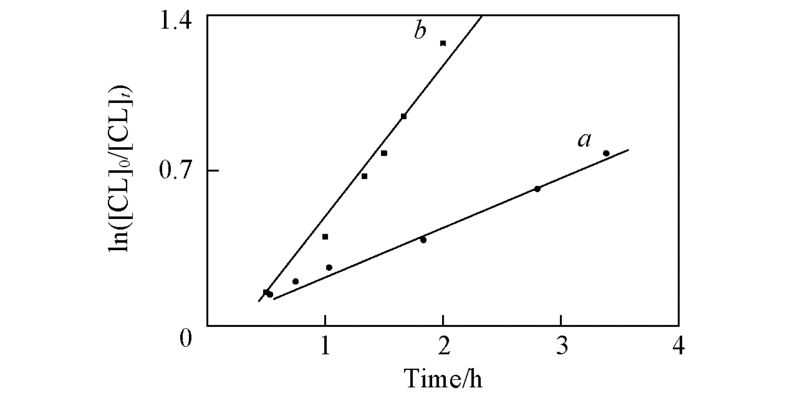
Fig.3 First-order kinetics plots for the ROP of ε-CL with 3a/2-propanol as catalyst/initiator in toluene with [CL]0=0.5 mol/L, [CL]/[Cat]=200:1 at 40 ℃(a) and 60 ℃(b)
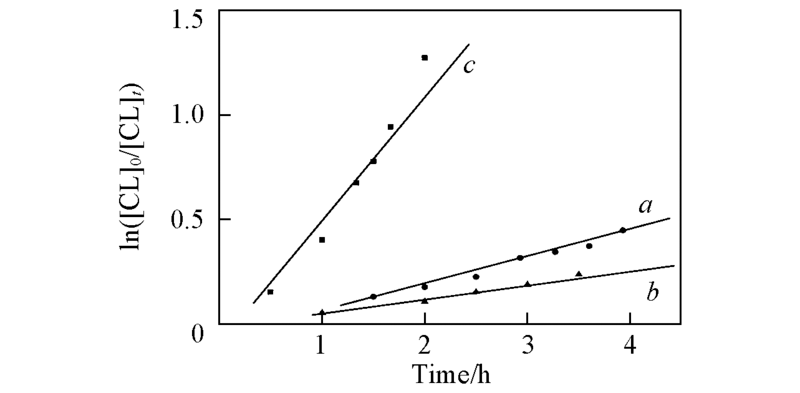
Fig.4 First-order kinetics plots for the ROP of ε-CL with 1a,3a/2-propanol as catalyst/initiator in toluene with 60 ℃, [CL]0=0.5 mol/L, [CL]/[Cat]=200:1 for 1a(a), 2a(b) and 3a(c)
| [1] | Okada M., Progress in Polymer Science, 2002, 27, 87—133 |
| [2] | Wu J. C., Yu T. L., Chen C. T., Lin C. C., Coordination Chemistry Reviews,2006, 250, 602—626 |
| [3] | Albertsson A. C., Varma I. K., Biomacromolecules,2003, 4, 1466—1486 |
| [4] | Dai W. F, Du. Z. Z., He Y. Y, Lang M. D., Chem. J. Chinese Universities,2009, 30(10), 2076—2081 |
| (戴炜枫, 杜征臻, 何月英, 郎美东.高等学校化学学报, 2009, 30(10), 2076—2081) | |
| [5] | Malcolm H.C., Huffman J. C., Khamphee P., J. Chem. Soc.,Dalton Trans., 2001, 222—224 |
| [6] | Zhong Z. Y., Dijkstra P. J., Birg C., Westerhausen M., Feijen J., Macromolecules,2001, 34, 3863—3868 |
| [7] | Chisholm M. H., Navarro-Llobet D., Simonsick W. J., Macromolecules,2001, 34, 8851—8857 |
| [8] | Antelmann B., Chisholm M. H., Iyer S. S., Huffman J. C., Navarro-Llobet D., Pagel M., Simonsick W. J., Zhong W. Q., Macromolecules,2001, 34, 3159—3175 |
| [9] | Chakraborty D., Chen E., Macromolecules,2002, 35, 13—15 |
| [10] | Yu R. C., Hung C. H., Huang J. H., Lee H. Y., Chen J. T., Inorg. Chem., 2002, 41, 6450—6455 |
| [11] | Dagorne S., Lavanant L., Welter R., Chassenieux C., Haquette P., Jaouen G., Organometallics,2003, 22, 3732—3741 |
| [12] | Takashima Y., Nakayama Y., Hirao T., Yasuda H., Harada A., Journal of Organometallic Chemistry,2004, 689, 612—619 |
| [13] | Cayuela J., Bounor L. V., Cassagnau P., Michel A., Macromolecules,2006, 39, 1338—1346 |
| [14] | Chmura A. J., Davidson M. G., Jones M. D., Lunn M. D., Mahon M. F., Johnson A. F., Khunkamchoo P., Roberts S. L., Wong S. S. F., Macromolecules,2006, 39, 7250—7257 |
| [15] | O'Keefe B. J., Breyfogle L. E., Hillmyer M. A., Tolman W. B., J. Am. Chem. Soc., 2002, 124, 4384—4393 |
| [16] | Walker D. A., Woodman T. J., Schormann M., Hughes D. L., Bochmann M., Organometallics,2003, 22, 797—803 |
| [17] | Sarazin Y., Schormann M., Bochmann M., Organometallics,2004, 23, 3296—3302 |
| [18] | Deshayes G. Frédéric A. G, Degée P., Verbruggen I., Biesemans M., Willem R., Dubois P., Chemistry: A European Journal,2003, 9, 4346—4352 |
| [19] | Kowalski A., Duda A., Penczek S., Macromolecules,2000, 33, 689—695 |
| [20] | Delbridge E.E., Nelson C. R., Skelton B. W., White A. H.,Dalton Trans., 2007, 143—153 |
| [21] | Zhu W. P., Tong X. W., Shen Z. Q., Chem. J. Chinese Universities,2007, 28(6), 1186—1188 |
| (朱蔚璞, 童晓薇, 沈之荃. 高等学校化学学报, 2007, 28(6), 1186—1188) | |
| [22] | Chen L., Chen X. S., Deng M. X., Jing X. B., Chem. Res. Chinese Universities,2005, 21(3), 340—344 |
| [23] | Nomura N., Aoyama T., Ishii R., Kondo T., Macromolecules,2005, 38(13), 5363—5366 |
| [24] | Benn R., Runska A., Lehmkuhl H., Janssen E., Kruger C., Angew.Chem. Int.Ed.Eng., 1983, 22, 779—780 |
| [25] | Qu Z., Li X., Pang X., Duan R. L., Gao B., Chen X. S., Chem. J. Chinese Universities,2014, 35(3), 626—632 |
| (曲智, 李想, 庞烜, 段然龙, 高波, 陈学思. 高等学校化学学报, 2014, 35(3), 626—632) | |
| [26] | Pang X., Du H. Z., Chen X. S., Wang X. H., Jing X. B., Chemistry: A European Journal,2008, 14, 3126—3136 |
| [1] | ZHANG Wanbin, WANG Yanmeng, WANG Shaowu, TONG Xin, HAN Xiaoqian, ZHANG Ce, ZHANG Guanghua, ZHU Xiuzhong. Synthesis of Poly(allyl glycidyl ether) Bearing Alkyl Functional Side Groups and Its Plasticizing and Antistatic Effects for PVC [J]. Chem. J. Chinese Universities, 2021, 42(9): 2961. |
| [2] | MA Yukun, JIN Hui, REN Chuanli, LI Zhibo. Ring-opening Polymerization of Cyclic Esters Using Recyclable Polystyrene Supported Urea-Base Binary Catalyst [J]. Chem. J. Chinese Universities, 2021, 42(9): 2968. |
| [3] | LI Rongye, NI Yunxia, LIU Dandan, LI Zhi, CHENG Yuxin, XIA Mingxin, FU Xiaohui. Synthesis and Characterization of Thermoresponsive Polypeptide/polypeptoid Block Copolymers [J]. Chem. J. Chinese Universities, 2021, 42(3): 850. |
| [4] | LI Chen, LI Yuesheng. Living Ring-opening Polymerization of O-Carboxyanhydrides Catalyzed by Pyridine Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(10): 3203. |
| [5] | MA Qian,WU Xiaohui,YU Lin,DING Jiandong. Design and Synthesis of Novel Iodinated Polycarbonates with Inherent X-Ray Opacity † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2233. |
| [6] | LI Juan, DU Fanfan, FENG Rui, HU Qian, JIE Suyun, LI Bogeng. Synthesis of Cyclic and Linear Block Copolyesters via Ring-opening Copolymerization of ε-Caprolatone and L-Lactide Catalyzed by Zinc Complex† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1297. |
| [7] | WANG Huijie, YU Haiyang, ZHANG Dawei, TANG Zhaohui, CAO Qi. Synthesis and Characterization of Poly(E,K) and Poly(E,R)† [J]. Chem. J. Chinese Universities, 2017, 38(1): 165. |
| [8] | WANG Lei, YANG Li, GAO Chengyong. Synthesis, Characterization of Methoxyl-bridged Aluminium-lithium Heterobimetallic Complex and Catalysis for the Ring-opening Polymerization of ε-Caprolactone† [J]. Chem. J. Chinese Universities, 2015, 36(4): 794. |
| [9] | SHI Xinbo, LI Lin, GAO Haiyang, WU Qing. Synthesis of Hyperbranched Polyethylene with Poly(ε-caprolactone) Arms and Its Self-assembly in n-Hexane [J]. Chem. J. Chinese Universities, 2015, 36(11): 2335. |
| [10] | LIANG Zhen-Hua, NI Xu-Feng*, SHEN Zhi-Quan. Controlled Ring\|opening Polymerization of ε-Caprolactone Initiated by in situ Formed Yttrium Tribenzyloxide Complex [J]. Chem. J. Chinese Universities, 2011, 32(8): 1881. |
| [11] | YANG Peng, GAO Ya-Juan, GUAN Yong, ZHENG An-Na*. Promoting Effect of Ethyl Acetate on Anionic Ring-opening Polymerization of l,3,5-Trimethyl-l,3,5-tris(3,3,3-trifluoropropyl)cyclotrisiloxane(D3F) [J]. Chem. J. Chinese Universities, 2011, 32(6): 1431. |
| [12] | ZHANG Qing-Huan, FU Ling-Yun, ZHANG Jun, YANG Xin-Lin*. Synthesis of a Novel Fullerene End-capped Poly(γ-benzyl L-glutamate) [J]. Chem. J. Chinese Universities, 2011, 32(1): 175. |
| [13] | QI Min-Hua, JIA Hui-Fang, LIU Zhi-Sheng, SHEN Qi, SHEN Zhi-Quan. High Effective Ring-open Polymerization of ε-Caprolactone Catalyzed by Bicycolopentadienyl Samarium(Ⅱ) Complex (C5H5)2Sm(THF) [J]. Chem. J. Chinese Universities, 2010, 31(12): 2518. |
| [14] | LI Xin, LING Jun*, LIU Jin-Zhi, TIAN Jiang-Chuang, SUN Wei-Lin, SHEN Zhi-Quan*. Monte Carlo Simulation and Chain Transfer Reaction in Ring-opening Polymerization of ε-Caprolactone Initiated by Rare Earth Aryloxides [J]. Chem. J. Chinese Universities, 2010, 31(11): 2293. |
| [15] | WU Qiu-Hua, WEI Tian-Zhu, LIANG Fang, SONG Xi-Ming, HAN Guang-Xi, ZHANG Guo-Lin*. Synthesis and Micellization of Polyacrylamide/Poly(γ-benzyl-L-glutamate) Graft Copolymer [J]. Chem. J. Chinese Universities, 2008, 29(8): 1650. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||