

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (6): 1152.doi: 10.7503/cjcu20140131
• Analytical Chemistry • Previous Articles Next Articles
ZHOU Rendan, LI Laisheng*( ), CHENG Biaoping, NIE Guizhen, ZHANG Hongfu
), CHENG Biaoping, NIE Guizhen, ZHANG Hongfu
Received:2014-02-24
Online:2014-06-10
Published:2014-03-11
Contact:
LI Laisheng
E-mail:lilaishengcn@163.com
Supported by:CLC Number:
TrendMD:
ZHOU Rendan, LI Laisheng, CHENG Biaoping, NIE Guizhen, ZHANG Hongfu. Preparation and Evaluation of a Novel 6-mono-Nitrophenylamino-β-cyclodextrin Bonded SBA-15 Chiral Stationary Phase for HPLC†[J]. Chem. J. Chinese Universities, 2014, 35(6): 1152.
| Compound | k1 | k2 | k3 | α | Rs | V(ACN)∶V(MeOH)∶V(TEA)∶V(HOAc) | λ/nm |
|---|---|---|---|---|---|---|---|
| Metoprolol | 1.93 | 2.28 | 1.18 | 1.75 | 95∶5∶2∶2 | 275 | |
| Alprenolol | 1.96 | 2.12 | 1.08 | 0.91 | 95∶5∶1∶1 | 270 | |
| Atenolol | 4.07 | 4.76 | 1.17 | 1.56 | 95∶5∶4∶4 | 275 | |
| Propranolol | 2.22 | 2.59 | 1.17 | 1.51 | 95∶5∶1.5∶1.5 | 290 | |
| Esmolol | 1.76 | 2.11 | 1.20 | 1.67 | 95∶5∶2∶2 | 275 | |
| Pindolol | 2.91 | 3.09 | 1.06 | 0.80 | 97∶3∶2∶2 | 263 | |
| Labetalol | 2.36 | 2.59 | 2.86 | 95∶5∶4∶4 | 306 | ||
| Nadolol | 2.91 | 3.10 | 3.46 | 95∶5∶4∶4 | 276 | ||
| Bisoprolol | 2.92 | 3.16 | 1.08 | 0.68 | 95∶5∶1∶1 | 272 | |
| Sotalol | 1.66 | 1.72 | 1.04 | 0.85 | 95∶5∶4∶4 | 275 | |
| Carteolol | 2.67 | 2.84 | 1.06 | 0.76 | 95∶5∶2∶2 | 254 | |
| Celiprolol | 1.99 | 2.10 | 1.06 | 0.84 | 95∶5∶2∶2 | 332 | |
| Arotinolol | 2.97 | 3.41 | 1.15 | 1.21 | 95∶5∶4∶4 | 316 | |
| Carvedilol | 2.43 | 2.84 | 1.17 | 1.58 | 95∶5∶2∶2 | 285 |
Table 1 Separation results for β-blockers on NCDSP
| Compound | k1 | k2 | k3 | α | Rs | V(ACN)∶V(MeOH)∶V(TEA)∶V(HOAc) | λ/nm |
|---|---|---|---|---|---|---|---|
| Metoprolol | 1.93 | 2.28 | 1.18 | 1.75 | 95∶5∶2∶2 | 275 | |
| Alprenolol | 1.96 | 2.12 | 1.08 | 0.91 | 95∶5∶1∶1 | 270 | |
| Atenolol | 4.07 | 4.76 | 1.17 | 1.56 | 95∶5∶4∶4 | 275 | |
| Propranolol | 2.22 | 2.59 | 1.17 | 1.51 | 95∶5∶1.5∶1.5 | 290 | |
| Esmolol | 1.76 | 2.11 | 1.20 | 1.67 | 95∶5∶2∶2 | 275 | |
| Pindolol | 2.91 | 3.09 | 1.06 | 0.80 | 97∶3∶2∶2 | 263 | |
| Labetalol | 2.36 | 2.59 | 2.86 | 95∶5∶4∶4 | 306 | ||
| Nadolol | 2.91 | 3.10 | 3.46 | 95∶5∶4∶4 | 276 | ||
| Bisoprolol | 2.92 | 3.16 | 1.08 | 0.68 | 95∶5∶1∶1 | 272 | |
| Sotalol | 1.66 | 1.72 | 1.04 | 0.85 | 95∶5∶4∶4 | 275 | |
| Carteolol | 2.67 | 2.84 | 1.06 | 0.76 | 95∶5∶2∶2 | 254 | |
| Celiprolol | 1.99 | 2.10 | 1.06 | 0.84 | 95∶5∶2∶2 | 332 | |
| Arotinolol | 2.97 | 3.41 | 1.15 | 1.21 | 95∶5∶4∶4 | 316 | |
| Carvedilol | 2.43 | 2.84 | 1.17 | 1.58 | 95∶5∶2∶2 | 285 |
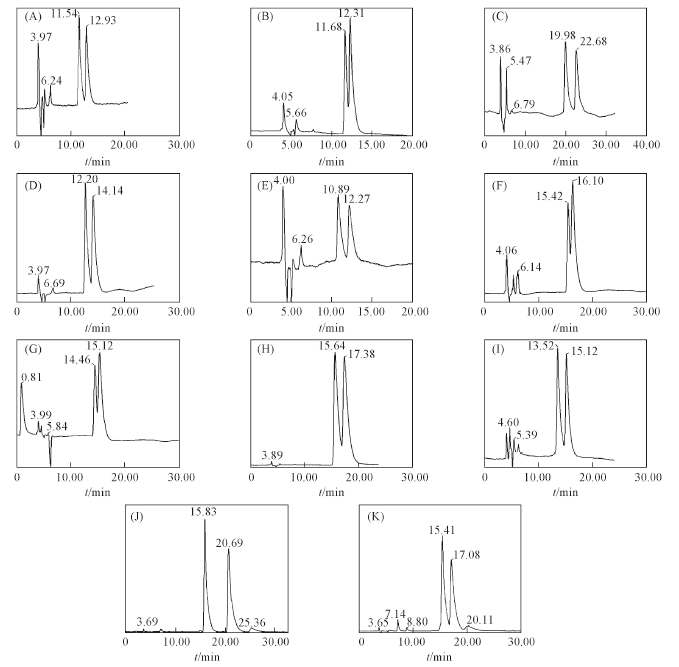
Fig.4 Some chiral chromatograms of β-blockers and dihydroflavanones on NCDSP(A) Metoprolol; (B) alprenolol; (C) atenolol; (D) propranolol; (E) esmolol; (F) pindolol; (G) carteolol; (H) arotinolol; (I) carvedilol; (J) 2'-hydroxy flavanone; (K) 4'-hydroxy flavanone.
| Compound | k1 | k2 | α | Rs | V(1%TEAA)∶V(ACN) | λ/nm |
|---|---|---|---|---|---|---|
| Flavanone | 3.97 | 4.26 | 1.07 | 0.69 | 80∶20 | 254 |
| 6-Methoxy flavanone | 3.77 | 3.95 | 1.05 | 0.54 | 80∶20 | 256 |
| 7-Methoxy flavanone | 3.69 | 3.95 | 1.07 | 0.86 | 80∶20 | 276 |
| 6-Hydroxy flavanone | 2.61 | 2.61 | 1.00 | 0.00 | 80∶20 | 256 |
| 2'-Hydroxy flavanone | 2.48 | 3.23 | 1.30 | 3.50 | 80∶20 | 254 |
| 4'-Hydroxy flavanone | 2.91 | 3.33 | 1.14 | 1.63 | 80∶20 | 254 |
Table 2 Separation results(Ⅰ) for dihydroflavanones on NCDSP in ACN mobile phase
| Compound | k1 | k2 | α | Rs | V(1%TEAA)∶V(ACN) | λ/nm |
|---|---|---|---|---|---|---|
| Flavanone | 3.97 | 4.26 | 1.07 | 0.69 | 80∶20 | 254 |
| 6-Methoxy flavanone | 3.77 | 3.95 | 1.05 | 0.54 | 80∶20 | 256 |
| 7-Methoxy flavanone | 3.69 | 3.95 | 1.07 | 0.86 | 80∶20 | 276 |
| 6-Hydroxy flavanone | 2.61 | 2.61 | 1.00 | 0.00 | 80∶20 | 256 |
| 2'-Hydroxy flavanone | 2.48 | 3.23 | 1.30 | 3.50 | 80∶20 | 254 |
| 4'-Hydroxy flavanone | 2.91 | 3.33 | 1.14 | 1.63 | 80∶20 | 254 |
| Compound | k1 | k2 | α | Rs | V(1%TEAA)∶V(MeOH) | λ/nm |
|---|---|---|---|---|---|---|
| Flavanone | 4.40 | 4.64 | 1.05 | 0.54 | 60∶40 | 254 |
| 6-Methoxy flavanone | 4.74 | 5.16 | 1.09 | 0.97 | 60∶40 | 256 |
| 7-Methoxy flavanone | 4.87 | 5.50 | 1.13 | 1.56 | 60∶40 | 276 |
| 6-Hydroxy flavanone | 2.78 | 2.99 | 1.08 | 0.85 | 60∶40 | 256 |
| 2'-Hydroxy flavanone | 3.03 | 4.25 | 1.40 | 5.21 | 60∶40 | 254 |
| 4'-Hydroxy flavanone | 3.40 | 3.40 | 1.00 | 0.00 | 60∶40 | 254 |
Table 3 Separation results(Ⅱ) for dihydroflavanones on NCDSP in MeOH mobile phase
| Compound | k1 | k2 | α | Rs | V(1%TEAA)∶V(MeOH) | λ/nm |
|---|---|---|---|---|---|---|
| Flavanone | 4.40 | 4.64 | 1.05 | 0.54 | 60∶40 | 254 |
| 6-Methoxy flavanone | 4.74 | 5.16 | 1.09 | 0.97 | 60∶40 | 256 |
| 7-Methoxy flavanone | 4.87 | 5.50 | 1.13 | 1.56 | 60∶40 | 276 |
| 6-Hydroxy flavanone | 2.78 | 2.99 | 1.08 | 0.85 | 60∶40 | 256 |
| 2'-Hydroxy flavanone | 3.03 | 4.25 | 1.40 | 5.21 | 60∶40 | 254 |
| 4'-Hydroxy flavanone | 3.40 | 3.40 | 1.00 | 0.00 | 60∶40 | 254 |
| Compound | k1 | k2 | α | Rs | V(1%TEAA)∶V(ACN)∶V(MeOH) | λ/nm |
|---|---|---|---|---|---|---|
| 6-Hydroxy flavanone | 3.18 | 3.32 | 1.04 | 0.51 | 70∶10∶20 | 256 |
| 4'-Hydroxy flavanone | 3.78 | 4.15 | 1.10 | 1.18 | 70∶10∶20 | 254 |
Table 4 Separation results(Ⅲ) for dihydroflavanones on NCDSP in ACN and MeOH mobile phase
| Compound | k1 | k2 | α | Rs | V(1%TEAA)∶V(ACN)∶V(MeOH) | λ/nm |
|---|---|---|---|---|---|---|
| 6-Hydroxy flavanone | 3.18 | 3.32 | 1.04 | 0.51 | 70∶10∶20 | 256 |
| 4'-Hydroxy flavanone | 3.78 | 4.15 | 1.10 | 1.18 | 70∶10∶20 | 254 |
| [1] | Chen L.R., Chiral Separation in Liquid Chromatography, Science Press, Beijing, 2006, 1—15 |
| (陈立仁. 液相色谱手性分离, 北京: 科学出版社, 2006, 1—15) | |
| [2] | Guo J. H., Chin. J. Cardiac Pacing and Electrophysiology, 2005, 19(5), 331—334 |
| (郭继鸿.中国心脏起搏与心电生理杂志, 2005,19(5), 331—334) | |
| [3] | Pei J. X., Wu P. S., Chin. J. Hypertens, 2011, 19(7), 608—610 |
| (裴静娴, 吴平生.中华高血压杂志, 2011,19(7), 608—610) | |
| [4] | Ali I., Gaitonde V. D., Aboul-Enein H. Y., Hussain A., Talanta, 2009, 78(2), 458—463 |
| [5] | Yu X. Y., Gu X. J., Xie Y., Qu S. L., Shen B. C., PTCA(Part B: Chem. Anal.), 2012, 48, 884—886 |
| (余小燕, 谷晓娟, 谢宇, 屈尚蓝, 沈报春. 理化检验-化学分册, 2012, 48, 884—886) | |
| [6] | Szente L., Szemán J., Anal. Chem., 2013, 85(17), 8024—8030 |
| [7] | Lai X. H., Ng S. C., J. Chromatogr. A, 2004, 1031, 135—142 |
| [8] | Poon Y. F., Muderawan I. W., Ng S. C., J. Chromatogr. A, 2006, 1101, 185—197 |
| [9] | Zhao D. Y., Huo Q. S., Feng J. L., Chmelka B. F., Stucky G. D., J. Am. Chem. Soc., 1998, 120(24), 6024—6036 |
| [10] | Li L. S., Ma H. P., Fang Y. S., Chen H., Chin. J. Anal. Lab., 2011, 30(11), 20—25 |
| (李来生, 马海萍, 方奕珊, 陈红.分析试验室, 2011,30(11), 20—25) | |
| [11] | Gao F., Zhao J. W., Zhang S., Zhou F., Jin W., Zhang X. M., Yang P. Y., Zhao D. Y., Chem. J. Chinese Universities, 2002, 23(8), 1494—1497 |
| (高峰, 赵建伟, 张松, 周峰, 金婉, 张祥民, 杨芃原, 赵东元.高等学校化学学报, 2002,23(8), 1494—1497) | |
| [12] | Zhou Z. M., Li X., Chen X. P., Hao X. Y., Anal. Chim. Acta, 2010, 678, 208—214 |
| [13] | Liu Y., Zhang Y.M., Sun S. X., Li Y. M., Chen R. T., J. Chem. Soc., Perkin Trans. 2, 1997, 1609—1613 |
| [14] | Wang Y., Young D. J., Tan T. T. Y., Ng S. C., J. Chromatogr. A, 2010, 1217, 5103—5108 |
| [15] | Jin Z.Y., Xu X. M., Chen H. Q., Li X. H., Cyclodextrin Chemistry-Preparation and Application, Chemical Industry Press, Beijing, 2009, 300—301 |
| (金征宇, 徐学明, 陈寒青, 李学红. 环糊精化学-制备与应用, 北京: 化学工业出版社, 2009, 300—301) | |
| [16] | Armstrong D. W., Chen S., Chang C., Chang S., J. Liq. Chromatogr. Rel. Technol., 1992, 15(3), 545—556 |
| [17] | Chang S. C., Reid Ⅲ G. L., Chen S., Chang C. D., Armstrong D. W., Trac-trend. Anal. Chem., 1993, 12(4), 144—153 |
| [18] | Li L. S., Zhou R. D., Cheng B. P., Nie G. Z., Zhang Y., J. Nanchang Univ.(Nat. Sci.), 2013, 37(2), 145—151 |
| (李来生, 周仁丹, 程彪平, 聂桂珍, 张扬. 南昌大学学报(理科版), 2013, 37(2), 145—151) | |
| [19] | Armstrong D. W., Chang C. D., Less S. H., J. Chromatogr. A, 1991, 539, 83—90 |
| [20] | Armstrong D. W., Li W., Chromatography, 1987, 2, 43—48 |
| [1] | WANG Mingfang, FU Hua, FU Zhibo, WANG Yuerong, ZHANG Hongyang, ZHANG Min, HU Ping. Separation and Characterization of Polymer Blends Using Online Ultra-high Performance Liquid Chromatography-Size Exclusion Chromatography [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210865. |
| [2] | LI Jing, SU Wei, WANG Xueyuan, FU Peng, SUN Yan. Synthesis and Characterization of Antihypertensive Drug Aranidipine and Its Related Impurities [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210663. |
| [3] | WANG Tianqi,YU Qiongwei,FENG Yuqi. Analysis of Imidazole Propionic Acid in Serum of Patients with Type 2 Diabetes Based on NiO@SiO2 Solid-phase Extraction Coupled with Liquid Chromatography-Mass Spectrometry † [J]. Chem. J. Chinese Universities, 2020, 41(2): 262. |
| [4] | ZHAO Mengxin, MENG Zhe, LI Heping, MA Zongqin, ZHAN Haijuan, LIU Wanyi. Photodegradation of Antibiotic in Environmental Water by Graphene Oxide Modulation Bismuth Molybdate Under Visible Light Irradiation [J]. Chem. J. Chinese Universities, 2020, 41(11): 2479. |
| [5] | ZHANG Hui, ZHANG Chenjie, XU Minmin, YUAN Yaxian, YAO Jianlin. Investigation on the Reaction of o-Aminothiophenol and 2-Iodobenzoyl Chloride Monitored by SERS-HPLC Technique [J]. Chem. J. Chinese Universities, 2020, 41(11): 2496. |
| [6] | SUN Haofan,ZHANG Lingyi,PATRICK Norman,ZHANG Weibing. Molecular Dynamic of Various DNA Sequences Binding of Dithienylethenes† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1229. |
| [7] | ZHAO Huanxi,WANG Qiuying,SUN Xiuli,LI Xue,MIAO Rui,WU Dongxue,LIU Shuying,XIU Yang. Discrimination of Ginseng Origins and Identification of Ginsenoside Markers Based on HPLC-MS Combined with Multivariate Statistical Analysis† [J]. Chem. J. Chinese Universities, 2019, 40(2): 246. |
| [8] | ZHOU Min, XU Xiaoying, LONG Yuande. Enantioseparation of Seventeen Kinds of β-Lactams on Carboxymethyl-β-cyclodextrin Chiral Stationary Phase and Research on Enantioseparation Mechanism [J]. Chem. J. Chinese Universities, 2018, 39(6): 1164. |
| [9] | QIU Xiuzhen, HUA Yongbiao, GUO Huishi, LU Wenguan. Preparation of a Molecularly Imprinted Polymer Nanotubes Membrane and Its Application in the Determination of Catecholamines in Urine Samples† [J]. Chem. J. Chinese Universities, 2018, 39(4): 653. |
| [10] | MIAO Rui,WU Dongxue,WANG Qiuying,ZHAO Huanxi,LI Xue,XIU Yang,LIU Shuying. Rapid Separation of Ginsenosides Based on Multi-walled Carbon Nanotubes† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2178. |
| [11] | ZHOU Xinhui, WANG Haishui. Co-effect of L-Cysteine Self-assembled Monolayers and Mixed Solvents on Chiral Separation of DL-glutamic Acid† [J]. Chem. J. Chinese Universities, 2017, 38(6): 1076. |
| [12] | LI Nan, ZHAO Huanxi, LI Jing, WANG Nan, YU Bohao, YUE Hao, YU Shanshan. Transformation of Total Notoginsenosides by Recombinant Endocellulase Fpendo5A† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2185. |
| [13] | CHENG Biaoping, LI Laisheng, ZHOU Rendan, LI Liang, ZHANG Hongfu. Enantioseparations of Triazole Chiral Pesticides on Two β-Cyclodextrin-bonded Stationary Phases with Different Linkages by HPLC† [J]. Chem. J. Chinese Universities, 2015, 36(5): 872. |
| [14] | TURSON Mamat, DAWUT Gulbahar, EMIN Risalat, CHU Ganghui, JELIL Mahmutjan, TURHON Muhetar. Preparation of Quercetin Imprinted Polymer by Living Radical Polymerization and Its Application in the Composition Analysis of Zukamu Granules for Uighur Medicine† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2402. |
| [15] | NIE Guizhen, LI Laisheng, CHENG Biaoping, ZHOU Rendan, ZHANG Hongfu. Enantioseparations of Dihydropyridine Drugs by Sulfobutyl Ether-β-Cyclodextrin Modified Capillary Electrochromatography† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1414. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||