

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 449.doi: 10.7503/cjcu20131247
• Articles: Inorganic Chemistry • Previous Articles Next Articles
WANG Yanyan1, ZHANG Zheng2, SUN Yanjun1, LI Jiyang1,*( )
)
Received:2013-12-19
Online:2014-03-10
Published:2019-08-01
Contact:
LI Jiyang
E-mail:lijiyang@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Yanyan, ZHANG Zheng, SUN Yanjun, LI Jiyang. Synthesis and Characterization of a New Open-framework Iron Phosphate†[J]. Chem. J. Chinese Universities, 2014, 35(3): 449.
| Empirical formula | Fe2P2 |
|---|---|
| Formula weight | 373.70 |
| Temperature/K | 293(2) |
| Wavelength/nm | 0.071073 |
| Crystal system, space group | Monoclinic, P21/c |
| a/nm | 0.97566(5) |
| b/nm | 0.98560(5) |
| c/nm | 1.24514(5) |
| β/(°) | 129.651(3) |
| V/nm3 | 0.92189(8) |
| Z, calculated density/(Mg·m-3) | 4, 2.693 |
| Absorption coefficient/mm-1 | 3.556 |
| F(000) | 744 |
| Crystal size | 0.15 mm×0.12 mm×0.10 mm |
| θ range for data collection/(°) | 2.71—28.31 |
| Limiting indices | -10≤h≤3 |
| -13≤k≤11 | |
| -16≤l≤16 | |
| Reflections collected/unique | 6615/2283(Rint=0.0606) |
| Completeness to θ=28.31 | 100.0% |
| Max. and min. transmission | 0.7175 and 0.6176 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2283/0/151 |
| Goodness-of-fit on F2 | 1.068 |
| Final R indices[I>2σ(I)] | R1=0.0338, wR2=0.0965 |
| R indices(all data) | R1=0.0372, wR2=0.0987 |
| Largest diff. peak and hole/(e·nm-3) | 1204, -721 |
Table 1 Crystal data and structure refinement for JU94*
| Empirical formula | Fe2P2 |
|---|---|
| Formula weight | 373.70 |
| Temperature/K | 293(2) |
| Wavelength/nm | 0.071073 |
| Crystal system, space group | Monoclinic, P21/c |
| a/nm | 0.97566(5) |
| b/nm | 0.98560(5) |
| c/nm | 1.24514(5) |
| β/(°) | 129.651(3) |
| V/nm3 | 0.92189(8) |
| Z, calculated density/(Mg·m-3) | 4, 2.693 |
| Absorption coefficient/mm-1 | 3.556 |
| F(000) | 744 |
| Crystal size | 0.15 mm×0.12 mm×0.10 mm |
| θ range for data collection/(°) | 2.71—28.31 |
| Limiting indices | -10≤h≤3 |
| -13≤k≤11 | |
| -16≤l≤16 | |
| Reflections collected/unique | 6615/2283(Rint=0.0606) |
| Completeness to θ=28.31 | 100.0% |
| Max. and min. transmission | 0.7175 and 0.6176 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2283/0/151 |
| Goodness-of-fit on F2 | 1.068 |
| Final R indices[I>2σ(I)] | R1=0.0338, wR2=0.0965 |
| R indices(all data) | R1=0.0372, wR2=0.0987 |
| Largest diff. peak and hole/(e·nm-3) | 1204, -721 |
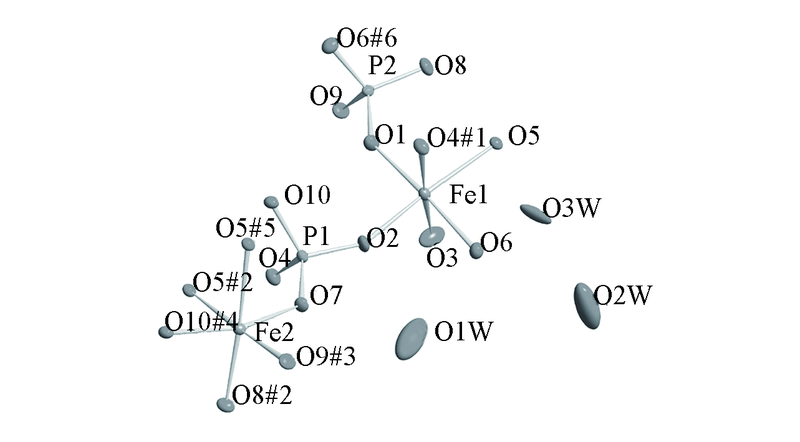
Fig.2 Thermal ellipsoids of JU94 given at 30% probabilitySymmetry codes: #1. -x+2, y-1/2, -z+3/2; #2. -x+2, y+1/2, -z+3/2; #3. x+1, y, z+1; #4. -x+3, -y+2, -z+2; #5. x+1, -y+3/2, z+1/2; #6. x, -y+3/2, z-1/2.
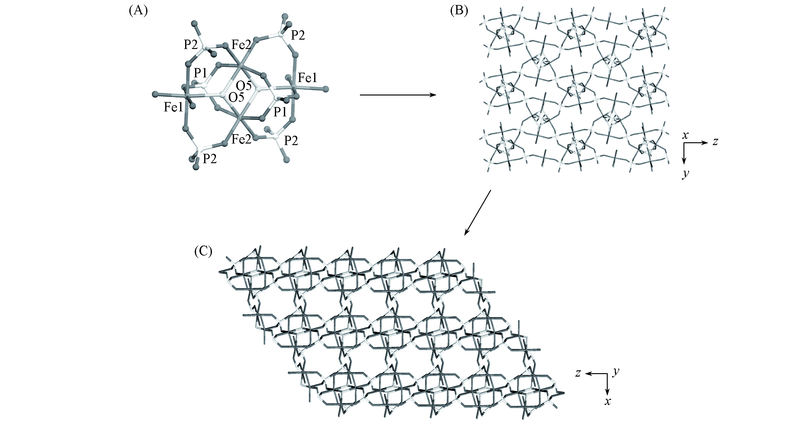
Fig.3 Tetranuclear iron cluster(Fe4P6O30H4) in JU94(A), the layer formed by clusters through sharing PO4 tetrahedra(B) and 3D framework constructed by the layers linked through O atoms(C)
| Absorption peak | IS/(mm·s-1) | QS/(mm·s-1) | HWHM/(mm·s-1) | Area fraction(%) |
|---|---|---|---|---|
| 1 | 0.40±0 | 0.36±0 | 0.19±0 | 51.4 |
| 2 | 0.42±0 | 0.67±0 | 0.18±0 | 48.6 |
Table 2 Parameters obtained in Mössbauer analysis*
| Absorption peak | IS/(mm·s-1) | QS/(mm·s-1) | HWHM/(mm·s-1) | Area fraction(%) |
|---|---|---|---|---|
| 1 | 0.40±0 | 0.36±0 | 0.19±0 | 51.4 |
| 2 | 0.42±0 | 0.67±0 | 0.18±0 | 48.6 |
| [1] | Wilson S. T., Lok B. M., Messina C. A., Marcus B. K., Patton R. L., Flanigen E. M., J. Am. Chem. Soc., 1982, 104, 1146—1147 |
| [2] | Lu H. Y., Tong X. Q., Yan Y., Yan W. F., Yu J. H., Xu R. R., Chem. J. Chinese Universities,2013, 34(7), 1571—1575 |
| (卢慧英, 仝晓强, 颜岩, 闫文付, 于吉红, 徐如人. 高等学校化学学报, 2013, 34(7), 1571—1575) | |
| [3] | Yu J. H., Xu R. R., Acc. Chem. Res., 2010, 43, 1195—1204 |
| [4] | Li Y., Yu J. H., Xu R. R., Baerlocher C., McCusker L. B., Angew. Chem. Int. Ed., 2008, 47, 4401—4405 |
| [5] | Li Y., Yu J. H., Xu R. R., Angew. Chem. Int. Ed., 2013, 52, 1673—1677 |
| [6] | Yu J. H., Xu R. R., Chem. Soc. Rev., 2006, 35, 593—604 |
| [7] | Song X., Li Y., Gan L., Wang Z., Yu J., Xu R., Angew. Chem. Int. Ed., 2008, 48, 314—317 |
| [8] | Rao C. N. R., Natarajan S., Choudhury A., Neeraj S., Ayi A. A., Acc. Chem. Res., 2001, 34, 80—87 |
| [9] | Yang G. Y., Sevov S. C., J. Am. Chem. Soc., 1999, 121, 8389—8390 |
| [10] | Thirumurugan A., Natarajan S., Dalton Trans., 2003, 17, 3387—3391 |
| [11] | Du Y., Yu J. H., Wang Y., Pan Q. H., Zou Y. C., Xu R. R., J. Solid State Chem., 2004, 177, 3032—3037 |
| [12] | Sassoye C., Marrot J., Loiseau T., Loiseau T., Férey G., Chem. Mater., 2002, 14, 1340—1347 |
| [13] | Beitone L., Marrot J., Loiseau T., Férey G., Henry M., Huguenard C., Gansmuller A., Taulelle F., J. Am. Chem. Soc., 2003, 125, 1912—1922 |
| [14] | Lin C. H., Wang S. N., Lii K. H., J. Am. Chem. Soc., 2001, 123, 4649—4650 |
| [15] | Soghomonian V., Chen Q., Haushalter R.C., Zubieta J., Charles J. O., Science, 199,259, 1596—1599 |
| [16] | Khan M. I., Meyer L. M., Haushalter R. C., Schweitzer A. L., Zubieta J., Dye J. L., Chem. Mater., 1996, 8, 43—53 |
| [17] | Murugavel R., Choudhury A., Walawalkar M. G., Pothiraja R., Rao C. N. R., Chem. Rev., 2008, 108, 3549—3655 |
| [18] | Choudhury A., Rao C. N. R., J. Struct. Chem., 2002, 43, 632—642 |
| [19] | Cavellec M., Grenèche J. M., Riou D., Férey G., Chem. Mater., 1998, 10, 2434—2439 |
| [20] | Cavellec M., Riou D., Férey G., Acta Crystallogr., 1994, C50, 1379—1381 |
| [21] | Choudhury A., Natarajan S., Rao C. N. R., Chem. Commun., 1999, 14, 1305—1306 |
| [22] | Armas S. F., Mesa J. L., Pizarro J. L., Garitaonandia J. S., Arriortua M. I., Rojo T., Angew. Chem. Int. Ed., 2004, 43, 977—980 |
| [23] | Rhule J. T., Hill C. L. , Jadd C. L., Schinazi R. F., Chem. Rev., 1998, 98, 327—358 |
| [24] | Rowan E., Nolte R. J. M., Angew. Chem. Int. Ed. Engl., 1998, 37, 63—68 |
| [25] | Kagan R., Mitzi D. B., Dimitrakopoulos C. D., Science,1999, 286, 945—947 |
| [26] | Knof U., Zelewsky A. V., Angew. Chem. Int. Ed. Engl., 1999, 38, 302—322 |
| [27] | Bruker AXS Inc., SAINT, Madison, WI, 2000 |
| [28] | Bruker AXS Inc., SHELXTL, Madison, WI, 2000 |
| [29] | Zima V., Lii K., J. Chem. Soc.,Dalton Trans., 1998, (24), 4109—4112 |
| [1] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| [2] | HONG Yangyu, XING Hongzhu, BING Qiming, GAO Xuwen, QI Bin, CHEN Yakun, SU Tan, ZOU Bo. Synthesis and Fluorescence Properties of Novel Ce3+-doped Manganese Phosphite Open-framework Materials [J]. Chem. J. Chinese Universities, 2021, 42(9): 2725. |
| [3] | ZHANG Huishuang, GAO Yanxiao, WANG Qiuxian, LI Xiangnan, LIU Wenfeng, YANG Shuting. High-low Temperature Properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 Cathode Material by Hydrothermal Synthesis with CTAB Assisted [J]. Chem. J. Chinese Universities, 2021, 42(3): 819. |
| [4] | WANG Ye, ZHANG Xiaosi, SUN Lijing, LI Bing, LIU Lin, YANG Miao, TIAN Peng, LIU Zhongyi, LIU Zhongmin. Morphology Control of SAPO Molecular Sieves under the Assistance of Organosilane [J]. Chem. J. Chinese Universities, 2021, 42(3): 683. |
| [5] | WANG Jianyu, ZHANG Qiang, YAN Wenfu, YU Jihong. Roles of Hydroxyl Radicals in Zeolite Synthesis [J]. Chem. J. Chinese Universities, 2021, 42(1): 11. |
| [6] | WU Qinming, WANG Yeqing, MENG Xiangju, XIAO Fengshou. Reconsideration of Crystallization Process for Aluminosilicate Zeolites [J]. Chem. J. Chinese Universities, 2021, 42(1): 21. |
| [7] | LIU Yabing,LI Mingyang,TIAN Ge,ALATENG Shaga,PEI Tonghe,NIE Jingsi. Syntheses, Structures and Catalytic Properties of Two Supramolecular Complexes Based on 2-Pyridylamine and Cluster † [J]. Chem. J. Chinese Universities, 2020, 41(5): 995. |
| [8] | ZHUO Mengning,LI Fei,JIANG Hao,CHEN Qianwen,LI Peng,WANG Lizhang. Preparation of SnO2/GDE Cathodes and Their Electrocatalytic Reduction of CO2 to Produce Formic Acid † [J]. Chem. J. Chinese Universities, 2020, 41(3): 530. |
| [9] | DONG Le, HUANG Xingliang, REN Junjie, DAI Xiaoping, LIU Zongyan, TIAN Hongfeng, WANG Zhidong, WU Xiaotong. Influence Mechanism of Particle Size and Distribution of Silica Sol in the Synthesis of Ferrierite Zeolite with High SiO2/Al2O3 Ratio [J]. Chem. J. Chinese Universities, 2020, 41(11): 2449. |
| [10] | ZHOU Hai, CHEN Hao, GUO Ya, KANG Min. Synthesis of Meso-porous Co3O4 Polyhedra and Their Electrochemical Properties† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1374. |
| [11] | GAO Ningxiao,XU Yulong,LIU Yong. Preparation of Carbon Dots from Soy Milk Powder and Fluorescent Nanofibers Containing Carbon Dots† [J]. Chem. J. Chinese Universities, 2019, 40(3): 555. |
| [12] | XIA Kun, ZHOU Dan, YANG Yun, YANG Shuijin, XIA Qinghua. Efficient Synthesis of Highly Uniform AlPO4-11 Microcrystalline and Study on the Crystallization Process† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1624. |
| [13] | SONG Wei, WANG Liqun, ZENG Shuangli, WANG Li, FAN Yong, XU Jianing. In situ Hydrothermal Synthesis, Crystal Structure and Fluorescence Properties of Two Cadmium Coordination Polymers† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1406. |
| [14] | XIA Kun,WANG Yi,ZHOU Dan,HUANG Zhe,WU Zhonghan,XIA Qinghua. Rapid Synthesis of CoSAPO-5 Zeolite and Efficiently Catalytic Epoxidation of α-Pinene with Air† [J]. Chem. J. Chinese Universities, 2018, 39(5): 941. |
| [15] | HUA Chenghe, MA Hongchao, DONG Xiaoli, ZHANG Xiufang. Synthesis and Photocatalytic Activity of α-Bi2O3 Nanotubes/Nitrogen Doped Carbon Quantum Dots Hybrid Material† [J]. Chem. J. Chinese Universities, 2018, 39(2): 200. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||