

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (6): 1229.doi: 10.7503/cjcu20130944
• Organic Chemistry • Previous Articles Next Articles
YUAN Wei1,2, REN Qingjiang1, SUN Hengda1,2, LI Hui1,*( ), CHENG Yanxiang1,*(
), CHENG Yanxiang1,*( ), MA Dongge1
), MA Dongge1
Received:2013-09-25
Online:2014-06-10
Published:2013-11-01
Contact:
LI Hui,CHENG Yanxiang
E-mail:lihui@ciac.ac.cn;yanxiang@ciac.ac.cn
Supported by:CLC Number:
TrendMD:
YUAN Wei, REN Qingjiang, SUN Hengda, LI Hui, CHENG Yanxiang, MA Dongge. Effect of Peripheral Substituents on Luminescent Properties of the Porphyrin Platinum(Ⅱ) Complexes†[J]. Chem. J. Chinese Universities, 2014, 35(6): 1229.
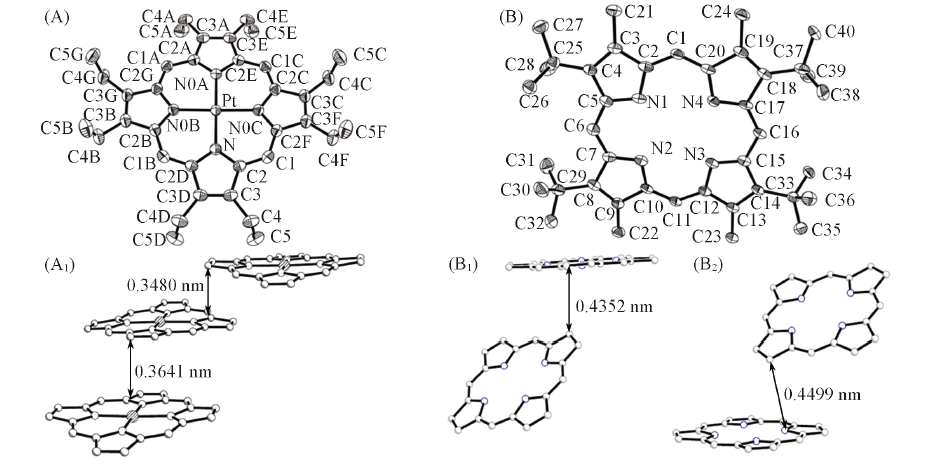
Fig.2 ORTEP drawings(30% probability ellipsoids)(A, B) and packing structures(A1, B1, B2) of PtTEMP(A, A1) and H2TBMP(B, B1, B2) H atoms are omitted for clarity.
| Compound | λabs/nm/lgεa | τb/μs | 10-4 | 10-4 | ||
|---|---|---|---|---|---|---|
| H2TEMP | 396/5.56; 497/4.55; 526/4.43; 568/4.32; 621/4.28 | 620, 689 | 0.017 | |||
| H2TBMP | 402/5.76; 501/4.65; 534/4.48; 572/4.35; 627/4.32 | 626, 691 | 0.021 | |||
| H2OMPP | 417/5.84; 512/4.57; 541/4.04; 589/4.11; 645/3.85 | 650, 709 | 0.019 | |||
| H2DMPP | 420/6.07; 514/4.81; 546/4.30; 590/4.30; 646/4.04 | 652, 711 | 0.015 | |||
| PtOEP | 378/5.49; 500/4.17; 534/4.87 | 648 | 74 | 0.45 | 0.61 | 0.74 |
| PtTEMP | 380/5.74; 500/4.23; 534/4.61 | 646 | 15 | 0.43 | 2.86 | 3.81 |
| PtTBMP | 380/5.88; 500/4.37; 534/4.78 | 656 | 34 | 0.58 | 1.71 | 1.23 |
| PtOMPP | 400/5.60; 508/4.58; 540/4.69 | 656, 712 | 40 | 0.29 | 0.73 | 1.78 |
| PtDMPP | 404/5.65; 510/4.28; 540/4.77 | 654, 710 | 57 | 0.36 | 0.63 | 1.12 |
Table 1 Photophysical data of porphyrin ligands and corresponding platinum(Ⅱ) complexes
| Compound | λabs/nm/lgεa | τb/μs | 10-4 | 10-4 | ||
|---|---|---|---|---|---|---|
| H2TEMP | 396/5.56; 497/4.55; 526/4.43; 568/4.32; 621/4.28 | 620, 689 | 0.017 | |||
| H2TBMP | 402/5.76; 501/4.65; 534/4.48; 572/4.35; 627/4.32 | 626, 691 | 0.021 | |||
| H2OMPP | 417/5.84; 512/4.57; 541/4.04; 589/4.11; 645/3.85 | 650, 709 | 0.019 | |||
| H2DMPP | 420/6.07; 514/4.81; 546/4.30; 590/4.30; 646/4.04 | 652, 711 | 0.015 | |||
| PtOEP | 378/5.49; 500/4.17; 534/4.87 | 648 | 74 | 0.45 | 0.61 | 0.74 |
| PtTEMP | 380/5.74; 500/4.23; 534/4.61 | 646 | 15 | 0.43 | 2.86 | 3.81 |
| PtTBMP | 380/5.88; 500/4.37; 534/4.78 | 656 | 34 | 0.58 | 1.71 | 1.23 |
| PtOMPP | 400/5.60; 508/4.58; 540/4.69 | 656, 712 | 40 | 0.29 | 0.73 | 1.78 |
| PtDMPP | 404/5.65; 510/4.28; 540/4.77 | 654, 710 | 57 | 0.36 | 0.63 | 1.12 |
| Compound | Von /V | Bmax/(cd·m-2) | ηc/(cd·A-1) | ηP/(lm·W-1) | EQE(%) | λmax/nm | CIE(x, y) |
|---|---|---|---|---|---|---|---|
| PtOEP | 4.0 | 271 | 2.14 | 1.98 | 5.8 | 648 | (0.71, 0.28) |
| PtTEMP | 4.2 | 247 | 1.73 | 1.56 | 4.3 | 648 | (0.70, 0.28) |
| PtTBMP | 4.0 | 412 | 2.21 | 1.98 | 6.3 | 648 | (0.71, 0.28) |
| PtOMPP | 4.3 | 457 | 0.42 | 0.36 | 1.7 | 665, 731 | (0.65, 0.27) |
| PtDMPP | 4.2 | 656 | 0.46 | 0.37 | 2.4 | 665, 733 | (0.52, 0.25) |
Table 2 EL performance of porphyrin platinum(Ⅱ) complexes(8% complexes in TCTA)*
| Compound | Von /V | Bmax/(cd·m-2) | ηc/(cd·A-1) | ηP/(lm·W-1) | EQE(%) | λmax/nm | CIE(x, y) |
|---|---|---|---|---|---|---|---|
| PtOEP | 4.0 | 271 | 2.14 | 1.98 | 5.8 | 648 | (0.71, 0.28) |
| PtTEMP | 4.2 | 247 | 1.73 | 1.56 | 4.3 | 648 | (0.70, 0.28) |
| PtTBMP | 4.0 | 412 | 2.21 | 1.98 | 6.3 | 648 | (0.71, 0.28) |
| PtOMPP | 4.3 | 457 | 0.42 | 0.36 | 1.7 | 665, 731 | (0.65, 0.27) |
| PtDMPP | 4.2 | 656 | 0.46 | 0.37 | 2.4 | 665, 733 | (0.52, 0.25) |
| [1] | Adachi C., Baldo M. A., Forrest S. R., Lamansky S., Thompson M. E., Kwong R. C., Appl. Phys. Lett., 2001, 78, 1622—1624 |
| [2] | Li H., Yuan W., Wang X. D., Cheng B., Cheng Y. X., Xie Z. Y., Wang L. X., Chinese J. Appl. Chem., 2012, 29(10), 1149—1157 |
| (李慧, 袁伟, 王兴东, 陈博, 程延祥, 谢志元, 王利祥.应用化学, 2012,29(10), 1149—1157) | |
| [3] | Kavitha J., Chang S. Y., Chi Y., Yu J. K., Hu Y. H., Chou P. T., Peng S. M., Lee G. H., Tao Y. T., Chien C. H., Carty A. J., Adv. Funct. Mater., 2005, 15, 223—229 |
| [4] | Dienel T., Proehl H., Fritz T., Leo K. J., Luminescence, 2004, 110, 253—257 |
| [5] | Kwong R. C., Lamansky S., Thompson M. E., Adv. Mater., 2000, 12, 1134—1138 |
| [6] | Nifiatis F., Su W., Haley J. E., Slagle J. E., Cooper T. M., J. Phys. Chem. A, 2011, 115, 13764—13772 |
| [7] | Kwong R. C., Sibley S., Dubovoy T., Baldo M., Forrest S. R., Thompson M. E., Chem. Mater., 1999, 11, 3709—3713 |
| [8] | Guo J. H., Wu Y., Ye K. Q., Sun Y. H., Wang Y., J. Mol. Sci., 2005, 21(1), 1—5 |
| (郭建华, 吴英, 叶开其, 孙迎辉, 王悦.分子科学学报, 2005,21(1), 1—5) | |
| [9] | Ikai M., Ishikawa F., Aratani N., Osuka A., Kawabata S., Kajioka T., Takeuchi H., Fujikawa H., Taga Y., Adv. Funct. Mater., 2006, 16, 515—519 |
| [10] | Luo K. J., Jiang S. P., Zhang L. F., Zhu W. G., Wang X., Chinese J. Appl. Chem., 2011, 28(10), 1155—1160 |
| (骆开均, 蒋世平, 张藜芳, 朱卫国, 王欣.应用化学, 2011,28(10), 1155—1160) | |
| [11] | Huo C., Zhang H. Y., Zhang P., Song W. F., Zhang H. D., Wang Y., Chem. J. Chinese Universities, 2007, 28(1), 71—74 |
| (霍城, 张红雨, 张萍, 宋伟峰, 张慧东, 王悦.高等学校化学学报, 2007,28(1), 71—74) | |
| [12] | Huo C., Zhang H. D., Zhang H. Y., Zhang H. Y., Yang B., Zhang P., Wang Y., Inorg. Chem., 2006, 45, 4735—4742 |
| [13] | Paine J. B., Kirshner W. B., Moskowitz D. W., Dolphin D., J. Org. Chem., 1976, 41, 3857—3860 |
| [14] | Ramaiah D., Karunakaran S. C., Vadakkancherit J. S., Tavarekere C. K., Alagar S., Pillai M. R., Nair S. A., Saras S. B., Rao M. C., Rao K. S., Process for the Preparation of Novel Porphyrin Derivatives and Their Use as PDT Agents and Fluorescence Probes US 20120308485Al, 2012-12-06 |
| [15] | Wohrle D., Adv. Mater., 1997, 9, 1191—1192 |
| [16] | Rothemund P., J. Am. Chem. Soc., 1936, 58, 625—627 |
| [17] | Adler A. D., Longo F. R., Finarelli J. D., Goldmacher J., Assour J., Korsakoff L., J. Org. Chem., 1967, 32, 476 |
| [18] | Milgrom L. R., Sheppard R. N., Slawin A. M. Z., Williams D. J., Polyhedron, 1988, 7, 57—61 |
| [19] | Hazell A., Acta Crystallogr. C, 1984, 40, 751—753 |
| [20] | Che C. M., Xiang H. F., Chui S. S., Xu Z. X., Roy V. A., Yan J. J., Fu W. F., Lai P. T., Williams I. D., Chem. Asian J., 2008, 3, 1092—1103 |
| [21] | Huang Q. M., Wang S. W., Li Q., Pan W., Deng P. X., Zhou H., Pan Z. Q., Chem. J. Chinese Universities, 2012, 33(4), 732—737 |
| (黄齐茂, 王司卫, 李清, 潘威, 邓鹏星, 周红, 潘志权.高等学校化学学报, 2012,33(4), 732—37) | |
| [22] | Wang K., Lin X., Wang X., Jia T., Zhang X. L., Zhang H., Ju X. L., Chem. J. Chinese Universities, 2012, 33(12), 2663—2669 |
| (王凯, 林笑, 万幸, 贾涛, 张秀兰, 张珩, 巨修练.高等学校化学学报, 2012,33(12), 2663—2669) | |
| [23] | Guo X. M., Guo B., Chen Y. H., Chem. J. Chinese Universities, 2013, 34(3), 499—502 |
| (郭喜明, 郭斌, 陈业宏.高等学校化学学报, 2013,34(3), 499—502) | |
| [24] | Vladimir C. S., Arie H. V., Victor G. A., Igor S. V., Tjeerd J. S., Holten D., J. Phys. Chem. B, 2000, 104, 9909—9917 |
| [25] | Pan S. L., Rothberg L. J., J. Am. Chem. Soc., 2005, 127, 6087—6094 |
| [26] | Wu W., Wu W., Ji S., Guo H., Wang X., Zhao J., Dyes Pigm., 2011, 89, 199—211 |
| [27] | Glauco P., Nick S., Michael B. A., Thomas N. L., J. Am. Chem. Soc., 1983, 105, 4639—4645 |
| [28] | Yersin H., Rausch A. F., Czerwieniec R., Hofbeck T., Fischer T., Coord. Chem. Rev., 2011, 255, 2622—2652 |
| [29] | Hou Q., Zhang Y., Li F. Y., Peng J. B., Cao Y., Organometallics, 2005, 24, 4509—4518 |
| [1] | MA Zhuoyuan, WANG Dayang. Status and Prospect of Surface Wettability of Molecular Self-assembled Monolayers [J]. Chem. J. Chinese Universities, 2021, 42(4): 1031. |
| [2] | LI Qing, YI Pinggui, TAO Hongwen, LI Yangyang, ZHANG Zhiyu, PENG Wenyu, LI Yuru. Solvent and Substituent Effects on Spectral Characteristics and Excited-state Intramolecular Proton Transfer of 2-(2-Aminophenyl) Benzothiazole† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1425. |
| [3] | ZHANG Wenqiang, ZHOU Jiadong, MA Weitao, ZHU Na, XIE Zengqi, LIU Linlin, MA Yuguang. Molecular Conformation and Aggregation Regulation of Bay-substituted Perylene Bismides: ortho-Methyl Steric Hindrance† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1757. |
| [4] | SI Pengfei, LUO Faliang, HAI Mei. Intermolecular Interactions and Crystallization and Melting Behavior of Poly(L-lactic acid)/4,4'-Thiobis Phenol Blends† [J]. Chem. J. Chinese Universities, 2015, 36(1): 188. |
| [5] | TANG Yinmin, HUO Yanping, HU Sheng, ZHANG Kun, ZHAO Fenghua, OUYANG Xinhua. Synthesis and Photoelectricity of Novel Zn Metal Complex Based on 8-Hydroxyquinoline with Thiophene Group† [J]. Chem. J. Chinese Universities, 2014, 35(1): 48. |
| [6] | LIU Peng, LI Shushi, WANG Changsheng. Effects of Substituents on the Binding Energy in Hydrogen-bonded Complexes Containing Adenine and Thymine† [J]. Chem. J. Chinese Universities, 2014, 35(1): 154. |
| [7] | MENG Su-Ci, YIN Xiu-Lian, MA Jing, XIE Ji-Min. Theoretical Studies on Solvent Effects and Intermolecular Interactions of Organic π-Conjugated Ligand in Solutions [J]. Chem. J. Chinese Universities, 2012, 33(11): 2492. |
| [8] | LI Peng, ZHANG Heng-Jun, ZHU Dong-Xia, SHAN Guo-Gang, LIAO Yi, SU Zhong-Min. Synthesis and Photo-electricity Properties of Hydroxyphenyl-pyrimidine Beryllium(Ⅱ) Complex [J]. Chem. J. Chinese Universities, 2012, 33(09): 2056. |
| [9] | SUN Yuan-Yuan, SHAN Ning, WANG Bin-Bin, LIAN Wen-Hui, YU Miao, SHI Tong-Shun. Synthesis and Characterization of Bis-porphyrins with Different Substituents [J]. Chem. J. Chinese Universities, 2012, 33(03): 496. |
| [10] | LIANG Xue, SUN Tao, WANG Yi-Bo. Symmetry-adapted Perturbation Theory Study on the Nature of Benzene-halogen(X2, X=F, Cl, Br, I) [J]. Chem. J. Chinese Universities, 2012, 33(03): 541. |
| [11] | ZHANG Peng, YANG Zhao-Yu, QIU Shu-Xuan, DING Ke-Yi, LUO Jian-Bin, XIE Xing-Yi. Synthesis and Characterization of Poly(ethylene glycol)/hydroxyapatite Hybrid Nanomaterials [J]. Chem. J. Chinese Universities, 2012, 33(01): 22. |
| [12] | YANG Gui-Xia, HUANG Zong-Hao*, YANG Pei-Pei, XIN Yi, JIANG Zi-Jiang, ....... Theoretical Studies on the Structural and Optoelectronic Properties of F-Substituted Silole Derivatives [J]. Chem. J. Chinese Universities, 2009, 30(11): 2274. |
| [13] | ZHAO Jian-Xin1, QIAO Yi-Tao1, FENG Jing1, LUO Zhao-Feng2, YUAN Zhi1*. Interaction of Copolymer-Zn with Polypeptide [J]. Chem. J. Chinese Universities, 2008, 29(3): 658. |
| [14] | WANG Fei-Jun1, SHAO Zi-Qiang1*, WANG Wen-Jun1, LÜ Shao-Yi1, FENG Zeng-Guo1, LIAO Bing2. Effect of Alkali Agents on Molecular Structure of PAC and Drilling-mud Fluid Loss [J]. Chem. J. Chinese Universities, 2008, 29(11): 2326. |
| [15] | CAO Yan1, LI Hui-Quan1*, ZHANG Yi1, ZHANG Jun2*, HE Jia-Song2. Synthesis of Cellulose Acetates with Low Degree of Substituent and Their Water Solubility [J]. Chem. J. Chinese Universities, 2008, 29(10): 2115. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||