

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (4): 895.doi: 10.7503/cjcu20130766
• Polymer Chemistry • Previous Articles
ZHOU Haifeng, YANG Dongjie, QIU Xueqing*( ), WU Xiaolei
), WU Xiaolei
Received:2013-08-06
Online:2014-04-10
Published:2014-02-27
Contact:
QIU Xueqing
E-mail:cexqqiu@scut.edu.cn
Supported by:CLC Number:
TrendMD:
ZHOU Haifeng, YANG Dongjie, QIU Xueqing, WU Xiaolei. Structural Characterization, Adsorption and Dispersion Properties of Sodium Lignosulfonate by Horseradish Peroxidase Incubation†[J]. Chem. J. Chinese Universities, 2014, 35(4): 895.
| Sample | Concentration of HRP/(g·L-1) | Incubation time/h | 10-3 Mn | 10-4 Mw | Mw/Mn |
|---|---|---|---|---|---|
| D748 | 0 | 0 | 4.8 | 1.40 | 2.92 |
| SL | 0 | 0 | 2.7 | 0.99 | 3.69 |
| HRP-SL 1 | 0.2 | 12 | 2.9 | 1.13 | 3.96 |
| HRP-SL 2 | 0.5 | 12 | 3.3 | 1.42 | 4.25 |
| HRP-SL 3 | 1 | 12 | 3.5 | 1.63 | 4.62 |
| HRP-SL 4 | 3 | 12 | 3.8 | 1.90 | 5.03 |
| HRP-SL 5 | 6 | 12 | 4.6 | 2.50 | 5.47 |
| HRP-SL 6 | 1 | 0.33 | 3.5 | 1.61 | 4.58 |
| HRP-SL 7 | 1 | 0.67 | 3.5 | 1.63 | 4.60 |
| HRP-SL 8 | 1 | 1.0 | 3.6 | 1.65 | 4.63 |
| HRP-SL 9 | 1 | 36 | 3.6 | 1.66 | 4.63 |
| HRP-SL 10 | 6 | 0.33 | 4.6 | 2.48 | 5.45 |
| HRP-SL 11 | 6 | 0.67 | 4.5 | 2.49 | 5.48 |
| HRP-SL 12 | 6 | 1.0 | 4.6 | 2.51 | 5.47 |
| HRP-SL 13 | 6 | 36 | 4.6 | 2.52 | 5.49 |
Table 1 Reaction conditions and molecular weight distribution of SL by HRP incubation
| Sample | Concentration of HRP/(g·L-1) | Incubation time/h | 10-3 Mn | 10-4 Mw | Mw/Mn |
|---|---|---|---|---|---|
| D748 | 0 | 0 | 4.8 | 1.40 | 2.92 |
| SL | 0 | 0 | 2.7 | 0.99 | 3.69 |
| HRP-SL 1 | 0.2 | 12 | 2.9 | 1.13 | 3.96 |
| HRP-SL 2 | 0.5 | 12 | 3.3 | 1.42 | 4.25 |
| HRP-SL 3 | 1 | 12 | 3.5 | 1.63 | 4.62 |
| HRP-SL 4 | 3 | 12 | 3.8 | 1.90 | 5.03 |
| HRP-SL 5 | 6 | 12 | 4.6 | 2.50 | 5.47 |
| HRP-SL 6 | 1 | 0.33 | 3.5 | 1.61 | 4.58 |
| HRP-SL 7 | 1 | 0.67 | 3.5 | 1.63 | 4.60 |
| HRP-SL 8 | 1 | 1.0 | 3.6 | 1.65 | 4.63 |
| HRP-SL 9 | 1 | 36 | 3.6 | 1.66 | 4.63 |
| HRP-SL 10 | 6 | 0.33 | 4.6 | 2.48 | 5.45 |
| HRP-SL 11 | 6 | 0.67 | 4.5 | 2.49 | 5.48 |
| HRP-SL 12 | 6 | 1.0 | 4.6 | 2.51 | 5.47 |
| HRP-SL 13 | 6 | 36 | 4.6 | 2.52 | 5.49 |
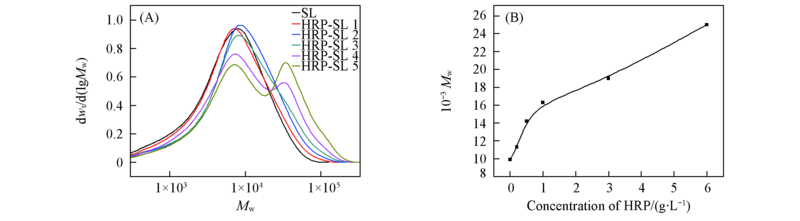
Fig.1 Effect of concentration of HRP on molecular weight distribution of SL by HRP incubation (A) Molecular weight distribution; (B) relationship of molecular weight and concentration of HRP.
| Sample | Concentration of phenolic group/(mmol·g-1) | Concentration of sulfonic group/(mmol·g-1) |
|---|---|---|
| SL | 2.35 | 1.30 |
| HRP-SL 6 | 1.72 | 1.42 |
| HRP-SL 7 | 1.70 | 1.43 |
| HRP-SL 8 | 1.74 | 1.42 |
| HRP-SL 3 | 1.70 | 1.44 |
| HRP-SL 9 | 1.71 | 1.41 |
| HRP-SL 10 | 1.33 | 1.64 |
| HRP-SL 11 | 1.32 | 1.63 |
| HRP-SL 12 | 1.32 | 1.61 |
| HRP-SL 5 | 1.34 | 1.64 |
| HRP-SL 13 | 1.32 | 1.65 |
Table 2 Phenolic and sulfonic group content of SL by HRP incubation
| Sample | Concentration of phenolic group/(mmol·g-1) | Concentration of sulfonic group/(mmol·g-1) |
|---|---|---|
| SL | 2.35 | 1.30 |
| HRP-SL 6 | 1.72 | 1.42 |
| HRP-SL 7 | 1.70 | 1.43 |
| HRP-SL 8 | 1.74 | 1.42 |
| HRP-SL 3 | 1.70 | 1.44 |
| HRP-SL 9 | 1.71 | 1.41 |
| HRP-SL 10 | 1.33 | 1.64 |
| HRP-SL 11 | 1.32 | 1.63 |
| HRP-SL 12 | 1.32 | 1.61 |
| HRP-SL 5 | 1.34 | 1.64 |
| HRP-SL 13 | 1.32 | 1.65 |
| Peak intensity ratio | Assignment | SL | HRP-SL 3 | HRP-SL 5 |
|---|---|---|---|---|
| A3436/A1512 | OH stretching in phenolic and aliphatic structures | 1.588 | 1.430 | 1.347 |
| A2937/A1512 | C—H vibration in —CH3 and —CH2 | 1.002 | 0.987 | 0.969 |
| A2850/A1512 | C—H vibration in CH3O— | 0.962 | 0.947 | 0.770 |
| A1703/A1512 | C=O vibration in unconjugated ketone, carbonyl and ester groups | 0.812 | 0.846 | 0.861 |
| A1604/A1512 | Aromatic skeleton vibrations | 1.242 | 1.181 | 1.174 |
| A1462/A1512 | C—H deformations band of asymmetric methyl and methylene | 0.948 | 0.921 | 0.872 |
| A1421/A1512 | Aromatic skeleton vibrations combined with C—H in plane deformations | 0.995 | 0.996 | 0.989 |
| A1366/A1512 | Aliphatic C—H stretching in methyl groups and phenolic hydroxyl | 0.961 | 0.906 | 0.842 |
| A1164/A1512 | G ring plus C—O stretching | 1.686 | 1.437 | 1.318 |
| A1076/A1512 | S ring and C—O stretching | 1.409 | 1.302 | 1.264 |
| A1044/A1512 | S=O stretching | 1.576 | 1.703 | 1.824 |
Table 3 IR bands assignment of SL and the ratio of characteristic peak at 1512 cm-1
| Peak intensity ratio | Assignment | SL | HRP-SL 3 | HRP-SL 5 |
|---|---|---|---|---|
| A3436/A1512 | OH stretching in phenolic and aliphatic structures | 1.588 | 1.430 | 1.347 |
| A2937/A1512 | C—H vibration in —CH3 and —CH2 | 1.002 | 0.987 | 0.969 |
| A2850/A1512 | C—H vibration in CH3O— | 0.962 | 0.947 | 0.770 |
| A1703/A1512 | C=O vibration in unconjugated ketone, carbonyl and ester groups | 0.812 | 0.846 | 0.861 |
| A1604/A1512 | Aromatic skeleton vibrations | 1.242 | 1.181 | 1.174 |
| A1462/A1512 | C—H deformations band of asymmetric methyl and methylene | 0.948 | 0.921 | 0.872 |
| A1421/A1512 | Aromatic skeleton vibrations combined with C—H in plane deformations | 0.995 | 0.996 | 0.989 |
| A1366/A1512 | Aliphatic C—H stretching in methyl groups and phenolic hydroxyl | 0.961 | 0.906 | 0.842 |
| A1164/A1512 | G ring plus C—O stretching | 1.686 | 1.437 | 1.318 |
| A1076/A1512 | S ring and C—O stretching | 1.409 | 1.302 | 1.264 |
| A1044/A1512 | S=O stretching | 1.576 | 1.703 | 1.824 |
| δ | Assignment | Amount(DMSO-1) | |
|---|---|---|---|
| SL | HRP-SL 5 | ||
| 7.75—7.20 | Aromatic protons in positions C-2 and C-6 with C=O group | 1.05 | 1.16 |
| 7.20—6.80 | H2, H5, H6 in guaiacyl units(G) | 1.00 | 0.83 |
| 6.80—6.20 | H2, H6 in syringyl units(S) | 0.56 | 0.51 |
| 5.75—5.25 | Hα, Hβ in β-β' structures | 0.89 | 1.06 |
| 5.00—4.75 | Hα in β-O-4' structures | 0.73 | 0.95 |
| 4.75—4.07 | Hβ, Hγ in β-O-4' structures | 2.74 | 4.69 |
| 4.00—3.50 | H in methoxyls | 5.66 | 3.57 |
| 3.20—2.90 | 0.61 | 0.76 | |
| 2.56—2.44 | DMSO | 1.00 | 1.00 |
| 2.40—2.10 | H in aromatic acetates | 0.50 | 0.58 |
| 1.95—1.85 | H in aliphatic acetates | 0.19 | 0.27 |
| 1.60—0.70 | Aliphatic H | 1.29 | 2.27 |
Table 4 Signal assignment in 1H NMR spectra of SL and results for quantification of functional groups(DMSO signal intensity as reference)
| δ | Assignment | Amount(DMSO-1) | |
|---|---|---|---|
| SL | HRP-SL 5 | ||
| 7.75—7.20 | Aromatic protons in positions C-2 and C-6 with C=O group | 1.05 | 1.16 |
| 7.20—6.80 | H2, H5, H6 in guaiacyl units(G) | 1.00 | 0.83 |
| 6.80—6.20 | H2, H6 in syringyl units(S) | 0.56 | 0.51 |
| 5.75—5.25 | Hα, Hβ in β-β' structures | 0.89 | 1.06 |
| 5.00—4.75 | Hα in β-O-4' structures | 0.73 | 0.95 |
| 4.75—4.07 | Hβ, Hγ in β-O-4' structures | 2.74 | 4.69 |
| 4.00—3.50 | H in methoxyls | 5.66 | 3.57 |
| 3.20—2.90 | 0.61 | 0.76 | |
| 2.56—2.44 | DMSO | 1.00 | 1.00 |
| 2.40—2.10 | H in aromatic acetates | 0.50 | 0.58 |
| 1.95—1.85 | H in aliphatic acetates | 0.19 | 0.27 |
| 1.60—0.70 | Aliphatic H | 1.29 | 2.27 |
| [1] | Ouyang X. P., Ke L. X., Qiu X. Q., Guo Y. X., Pang Y. X., J. Disper. Sci. Technol., 2009, 30(1), 1—6 |
| [2] | Qiu X. Q., Kong Q., Zhou M. S., Yang D. J., J. Phys. Chem. B,2010, 114(48), 15857—15861 |
| [3] | Yang D. J., Qiu X. Q., Pang Y. X., Zhou M. S., J. Disper. Sci. Technol., 2008, 29(9), 1296—1303 |
| [4] | Zhou M. S., Qiu X. Q., Yang D. J., Lou H. M., J. Disper. Sci. Technol., 2006, 27(6), 851—856 |
| [5] | Yan M. F., Qiu X. Q., Yang D. J., Hu W. L., Chem. J. Chinese Universities,2008, 29(11), 2312—2316 |
| (严明芳, 邱学青, 杨东杰, 胡文莉. 高等学校化学学报, 2008, 29(11), 2312—2316) | |
| [6] | Qiu X. Q., Lou H. M., Yang D. J., Pang Y. X., Fine Chem., 2005, 22(3), 161—167 |
| (邱学青, 楼宏铭, 杨东杰, 庞煜霞. 精细化工, 2005, 22(3), 161—167) | |
| [7] | Kobayashi S., Uyama H., Kimura S., Chem. Rev., 2001, 101(12), 3793—3818 |
| [8] | Veitch N. C., Phytochemistry, 2004, 65(3), 249—259 |
| [9] | Choi Y. J., Chae H. J., Kim E. Y., J. Biosci. Bioeng., 1999, 88(4), 368—373 |
| [10] | Guerra A., Ferraz A., Enzyme Microb. Technol., 2001, 28(4), 308—313 |
| [11] | Dordick J. S., Marletta M. A., Klibanov A. M., Biotechnol. Bioeng., 2004, 30(1), 31—36 |
| [12] | Blinkovsky A. M., Dordick J. S., J. Polym. Sci., Part A: Polym. Chem., 1993, 31(7), 1839—1846 |
| [13] | Liu J., Li L., Cheng J., Wang L., Ye L., J. Appl. Polym. Sci., 2001, 81(10), 2408—2418 |
| [14] | Tobimatsu Y., Takano T., Kamitakahara H., Nakatsubo F., J. Wood Chem. Technol., 2008, 28(2), 69—83 |
| [15] | Saake B., Argyropoulos D.S., Beinhoff O., Faix O., Phytochemistry,1996, 43(2), 499—507 |
| [16] | Cathala B., Saake B., Faix O., Monties B., J. Chromatogr. A,2003, 1020(2), 229—239 |
| [17] | Boerjan W., Ralph J., Baucher M., Annu. Rev. Plant Biol., 2003, 54(1), 519—546 |
| [18] | Sasaki S., NishidaT., Tsutsumi Y., Kondo R., FEBS Lett., 2004, 562(1), 197—201 |
| [19] | Tobimatsu Y., Takano T., Kamitakahara H., Nakatsubo F., J. Wood Sci., 2010, 56(3), 233—241 |
| [20] | Grönqvist S., Viikari L., Niku-Paavola M. L., Orlandi M., Canevali C., Buchert J., Appl. Microbiol. Biotechnol., 2005, 67(4), 489—494 |
| [21] | Zhou H. F., Yang D. J., Wu X. L., Qiu X. Q., Chem. J. Chinese Universities,2012, 33(1), 218—224 |
| (周海峰, 杨东杰, 伍晓蕾, 邱学青. 高等学校化学学报, 2012, 33(1), 218—224) | |
| [22] | Yang D.J., Zhou H. F., Xie S. Q., Wu X. L., Qiu X. Q.,Acta Polymerica Sinica, 2013, (2), 232—240 |
| (杨东杰, 周海峰, 谢绍朐, 伍晓蕾, 邱学青. 高分子学报, 2013, (2), 232—240) | |
| [23] | Childs R. E., Bardsley W. G., Biochem. J., 1975, 145(1), 93 |
| [24] | Qiu X.Q., Wu Y., Deng Y. H., Yang D. J., Ouyang X. P., Yi C. H.,Acta Polymerica Sinica, 2010, (6), 699—704 |
| (邱学青, 吴渊, 邓永红, 杨东杰, 欧阳新平, 易聪华. 高分子学报, 2010, (6), 699—704) | |
| [25] | De Sousa F., Reimann A., BjöRklund Jansson M., Nilberbrant N., The 11th International Symposium on Wood and Pulping Chemistry(ISWPC), Nice France,2001, 3, 649—653 |
| [26] | Li P. W., Yang D. J., Lou H. M., Qiu X. Q., Journal of Fuel Chemistry and Technology,2008, 36(5), 524—529 |
| (李朋伟, 杨东杰, 楼宏铭, 邱学青. 燃料化学学报, 2008, 36(5), 524—529) | |
| [27] | Yang D. J., Qiu X. Q., Zhou M. S., Lou H. M., Energy Convers. Manage., 2007, 48(9), 2433—2438 |
| [28] | Matsushita Y., Yasuda S., Bioresour. Technol., 2005, 96(4), 465—470 |
| [29] | Martínez A., Almendros G., González-Vila F., Fründ R., Solid State Nucl. Magn. Reson., 1999, 15(1), 41—48 |
| [30] | Tejado A., Pena C., Labidi J., Echeverria J., Mondragon I., Bioresour. Technol., 2007, 98(8), 1655—1663 |
| [31] | Yuan T. Q., He J., Xu F., Sun R. C., Polym. Degrad. Stabil., 2009, 94(7), 1142—1150 |
| [32] | Sun R., Mott L., Bolton J., J. Agric. Food Chem., 1998, 46(2), 718—723 |
| [33] | Boeriu C. G., Bravo D., Gosselink R. J. A., van Dam J. E. G., Ind. Crop. Prod., 2004, 20(2), 205—218 |
| [34] | Shao L., Qiu J., Feng H., Liu M., Zhang G., An J., Gao C., Liu H., Synth. Met., 2009, 159(17), 1761—1766 |
| [35] | Nugroho Prasetyo E., Kudanga T., Østergaard L., Rencoret J., Gutiérrez A., Del Río J.C., Ignacio Santos J., Nieto L., Jiménez-Barbero J., Martínez A. T., Bioresour. Technol., 2010, 101(14), 5054—5062 |
| [36] | Jahan M. S., Chowdhury D., Islam M. K., Moeiz S., Bioresour. Technol., 2007, 98(2), 465—469 |
| [37] | Kubo S., Kadla J. F., Macromolecules,2004, 37(18), 6904—6911 |
| [38] | Balakshin M., Capanema E., Chen C. L., Gratzl J., Kirkman A., Gracz H., J. Mol. Catal. B: Enzym., 2001, 13(1), 1—16 |
| [39] | Toledano A., Serrano L., Garcia A., Mondragon I., Labidi J., Chem. Eng. J., 2010, 157(1), 93—99 |
| [40] | Deng Y. H., Wu Y., Feng X. J., Ouyang X. P., Yang D. J., Qiu X. Q., Journal of South China University of Technology(Natural Science), 2010, 38(11), 74—79 |
| (邓永红, 吴渊, 冯鑫佳, 欧阳新平, 杨东杰, 邱学青. 华南理工大学学报(自然科学版), 2010, 38(11), 74—79) | |
| [41] | Boisvert J. P., Persello J., Castaing J. C., Cabane B., Colloids Surf., A: Physicochem. Eng. Aspects,2001, 178(1), 187—198 |
| [42] | Farrokhpay S., Morris G. E., Fornasiero D., Self P., J. Colloid Interface Sci., 2004, 274(1), 33—40 |
| [43] | Yi C. H., Qiu X. Q., Yang D. J., Lou H. M., CIESC Journal,2009, 60(4), 959—964 |
| (易聪华, 邱学青, 杨东杰, 楼宏铭. 化工学报, 2009, 60(4), 959—964) | |
| [44] | Yi C.H., Study of Corrosion Inhibition Performances of Lignosulfonate and Its Action Mechanism, South China University of Technology, Guangzhou, 2005 |
| (易聪华. 木质素磺酸盐的缓蚀性能及作用机理研究, 广州: 华南理工大学, 2005) | |
| [45] | Guo W.Y., Yang D. J., Li R., Qiu X. Q.,Acta Polymerica Sinica, 2012, (9), 988—996 |
| (郭闻源, 杨东杰, 李荣, 邱学青. 高分子学报, 2012, (9), 988—996) | |
| [46] | Ye D. Z., Jiang X. C., Xia C., Liu L., Zhang X., Carbohydr. Polym., 2012, 89, 876—882 |
| [47] | Chen R., Kokta B. V., Valade J. L., J. Appl. Polym. Sci., 1980, 25, 2211—2220 |
| [1] | ZHU Haotian, JIN Meixiu, TANG Wensi, SU Fang, LI Yangguang. Properties of Transition Metal-biimidazole-Dawson-type Tungstophosphate Hybrid Compounds as Supports for Enzyme Immobilization [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220328. |
| [2] | MA Chao, LIU Xiaona, NIE Chenyang, CHEN Lu, TIAN Peng, XU Hongyi, GUO Peng, LIU Zhongmin. Applications of X-ray and Electron Crystallography in Structural Investigations of Zeolites [J]. Chem. J. Chinese Universities, 2021, 42(1): 188. |
| [3] | GAO Xia,PAN Huibin,QIAO Chengfang,CHEN Fengying,ZHOU Yuan,YANG Wenhua. Construction of HRP Immobilized Enzyme Reactor Based on Hierarchically Porous Metal-organic Framework and Its Dye Degradation Application† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1591. |
| [4] | ZHAO Xingling, QI Guodong, WANG Qiang, CHU Yueying, GAO Wei, LI Shenhui, XU Jun, DENG Feng. Structure, Nature and Activity of Ga Species for Propane Aromatization in Ga/ZSM-5 Revealed by Solid-state NMR Spectroscopy [J]. Chem. J. Chinese Universities, 2020, 41(12): 2681. |
| [5] | TENG Yu,YANG Shaoming,BAI Chaopeng,ZHANG Jian. Preparation of HRP Molecularly Imprinted Electrochemical Sensor with Multi-walled Carbon Nanotubes as Sensitizing Materials and the Detection of H2O2 † [J]. Chem. J. Chinese Universities, 2020, 41(1): 78. |
| [6] | FAN Hongliang, ZHANG Tao, JIN Wei, JIN Qinhan. Novel Label-free Assay of Telomerase Based on DNAzyme† [J]. Chem. J. Chinese Universities, 2015, 36(4): 625. |
| [7] | QU Jian-Ying, KANG Shi-Ping, LOU Tong-Fang, DU Xue-Ping. Detection of H2O2 at Composite Membrane of [bmim] FeCl4 and PVA Loading HRP Modified Graphene/nano Au [J]. Chem. J. Chinese Universities, 2013, 34(9): 2097. |
| [8] | WEI Yong-Ji, LI Shun-Lai, YU Ming-Wu, DU Hong-Guang. Synthesis of 6-Amino-2-alkylthio Adenosine and Their Anti-platelet Aggregation Activity [J]. Chem. J. Chinese Universities, 2013, 34(11): 2524. |
| [9] | ZHOU Hai-Feng, YANG Dong-Jie, WU Xiao-Lei, QIU Xue-Qing. Structure and Adsorption Characterization of Sodium Lignosulfonate by Laccase Modification [J]. Chem. J. Chinese Universities, 2013, 34(1): 218. |
| [10] | SU Man-Xiu, WANG Li-Feng, DAI Zhi-Jun, YUAN Zhe-Ming, BAI Lian-Yang. Primary Structural Characterizations of Polypeptide and Antimicrobial Peptides QSAM Modeling [J]. Chem. J. Chinese Universities, 2012, 33(11): 2526. |
| [11] | LU Hai-Xia, WU Zai-Sheng, JIN Xiao-Yong, SHEN Guo-Li*, YU Ru-Qin. Novel Electrochemical Sensor Based on Seminetwork-type Au Nanoparticles Labled Horseradish Peroxidase [J]. Chem. J. Chinese Universities, 2009, 30(2): 263. |
| [12] | YAN Ming-Fang, QIU Xue-Qing*, YANG Dong-Jie, HU Wen-Li. Separation and Purification of Lignosulfonate [J]. Chem. J. Chinese Universities, 2008, 29(11): 2312. |
| [13] | JIANG Zhi-Liang*, LI Jian-Fu, LIANG Ai-Hui, LI Ji-Shun, TANG Ya-Fang, WANG Su-Mei, ZHANG Nan-Nan. Rayleigh Scattering Spectral Determination of Ultratrace Horseradish Peroxidase Based on o-Phenylenediamine Particles and Its Application [J]. Chem. J. Chinese Universities, 2008, 29(10): 1973. |
| [14] | FENG Xia-Guang, ZHANG Min, ZHAO Hu, WANG Huai-You*. Determination of Vancomycin in Injection via Enzyme Catalysis-Fluorescence Quenching Method [J]. Chem. J. Chinese Universities, 2007, 28(7): 1270. |
| [15] | DU Chuang1, WANG Chun-Yu2, ZHANG Guo1, TANG Jun2*. Molecular Weight and Molecular Weight Distribution Control of Ring-opening Metathesis Polymerization of Norbornene [J]. Chem. J. Chinese Universities, 2007, 28(10): 2018. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||