

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (5): 20220732.doi: 10.7503/cjcu20220732
收稿日期:2022-11-25
出版日期:2023-05-10
发布日期:2023-01-03
通讯作者:
严大峰
E-mail:dafengyan@hnu.edu.cn
基金资助:
YAN Dafeng1( ), XIE Chao2, CHEN Chen3
), XIE Chao2, CHEN Chen3
Received:2022-11-25
Online:2023-05-10
Published:2023-01-03
Contact:
YAN Dafeng
E-mail:dafengyan@hnu.edu.cn
Supported by:摘要:
电化学水裂解制备氢气因其固有的优势受到了广泛关注. 然而, 阳极氧气析出反应动力学缓慢、 能耗高, 极大地限制了其应用. 与氧析出反应不同, 一些无机化学品的电氧化无论是热力学还是动力学上都更易发生. 因此, 耦合氢气析出反应和无机化学品氧化在提高电化学制氢效率方面表现出巨大潜力. 与氧气析出反应相比, 无机化学品氧化可以显著降低过电位. 同时, 还可以在阳极去除污染物或制备高附加值化学品. 本综合评述总结了电化学制备氢气耦合无机化学品电氧化方面的研究进展. 首先, 介绍并讨论一些具有代表性的无机化学替代品, 如含氮的肼、 一氧化氮以及含硫的硫化氢、 二氧化硫等, 其可以实现在很低的电压下制备氢气并且从根本上避免氧气的产生. 另外, 引入电化学中和能能够进一步降低电化学制备氢气电解槽的槽压, 甚至可以实现在制备氢气的同时输出电力. 最后, 对该领域面临的挑战以及未来发展进行了展望.
中图分类号:
TrendMD:
严大峰, 谢超, 陈晨. 电化学氢气制备耦合无机化学品电氧化的研究进展. 高等学校化学学报, 2023, 44(5): 20220732.
YAN Dafeng, XIE Chao, CHEN Chen. Recent Progress on Strategies for Electrochemical Hydrogen Production Coupling with Oxidation of Inorganic Chemicals. Chem. J. Chinese Universities, 2023, 44(5): 20220732.
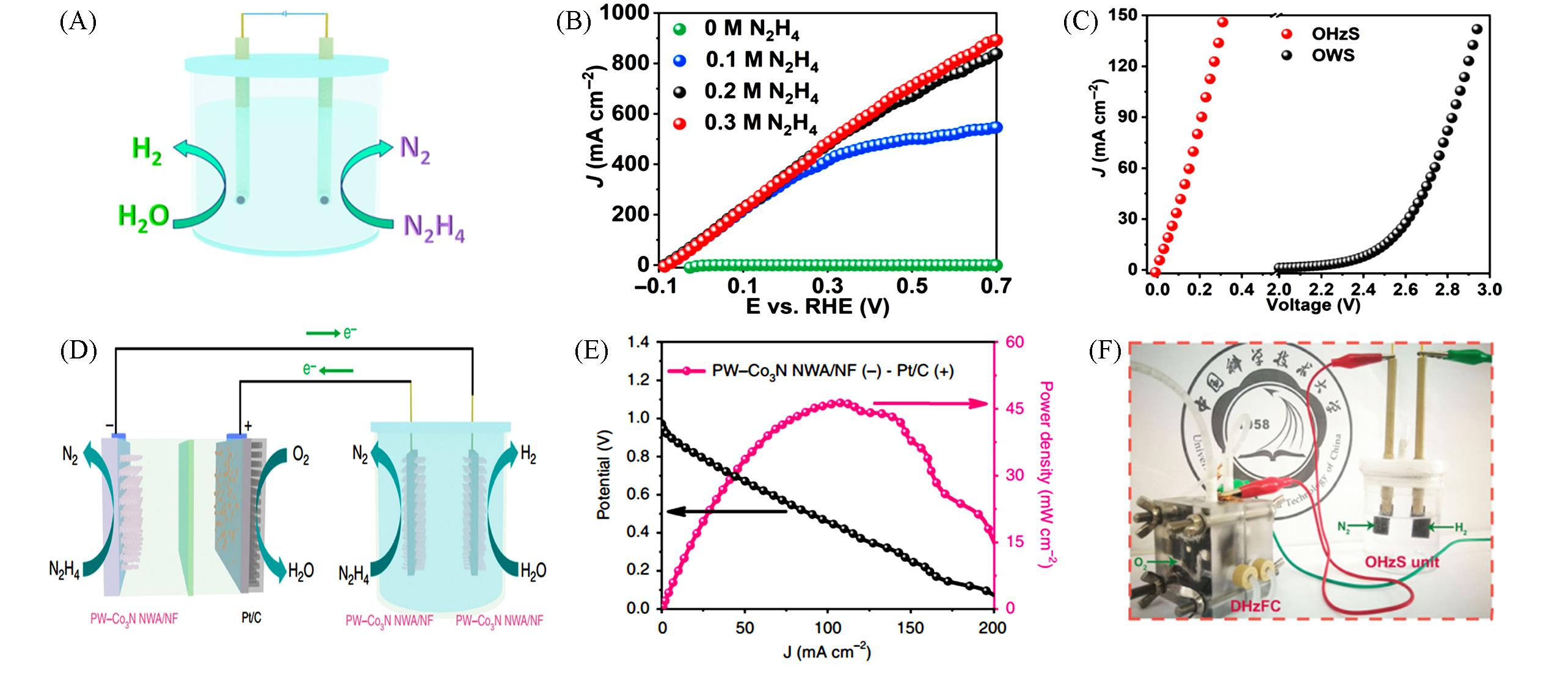
Fig.2 A schematic diagram of the electrochemical N2H4 oxidation coupling with H2 production(A), LSV curves of RuP2⁃CPM in 1.0 mol/L KOH with different concentrations of N2H4(B), comparison of LSV curves for N2H4 oxidation coupling with HER and traditional overall water splitting systems(C) [ 59], schematic illustration of a self⁃powered H2 production system integrating a direct hydrazine fuel cell (DHzFC) and an OHzS(D), the current density⁃voltage and current density⁃power density plots for the designed DHzFC(E), optical image of the designed self⁃powered H2 production system(F) [ 63]
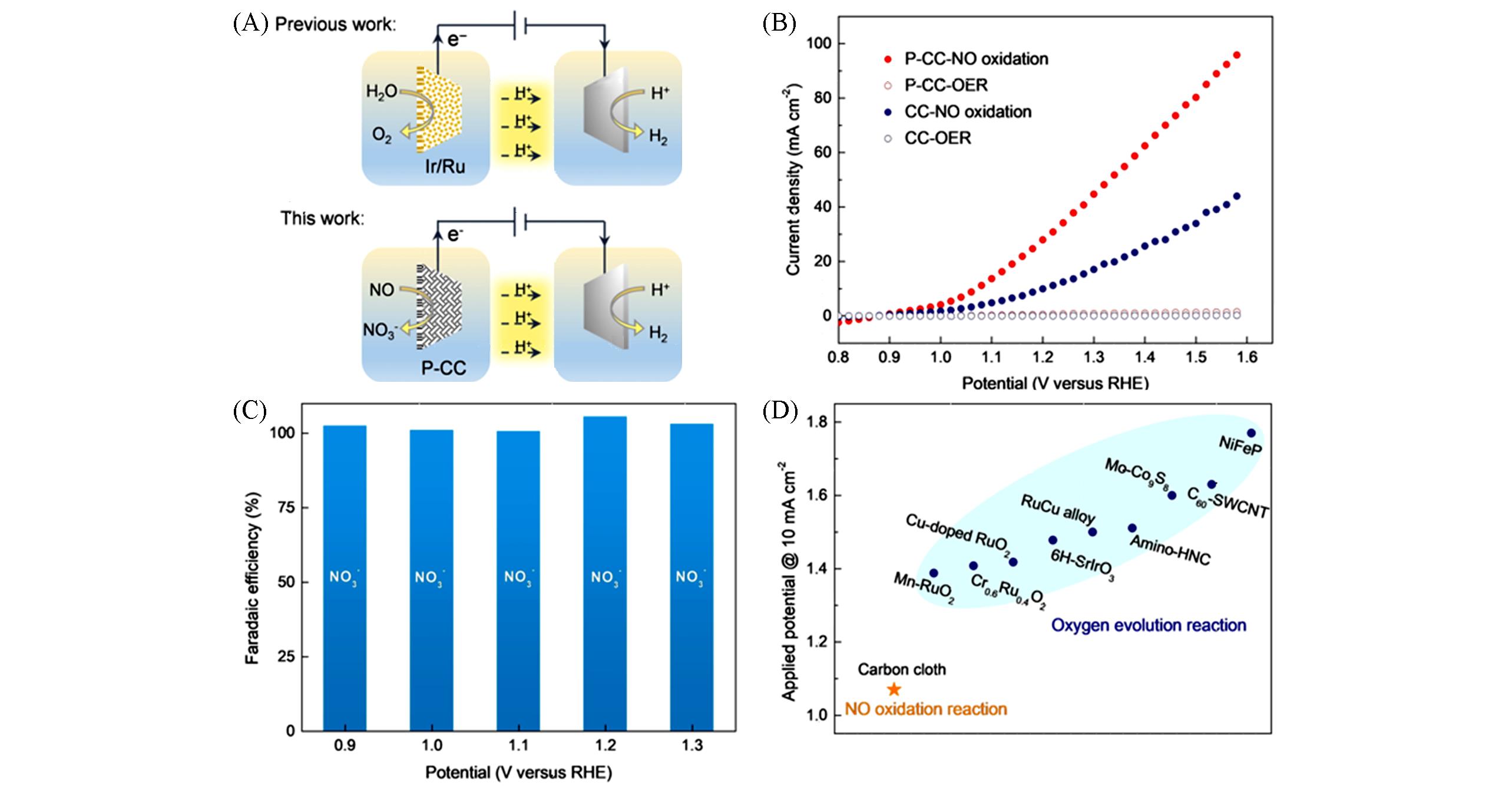
Fig.3 A schematic diagram of the electrochemical NO oxidation coupling with H2 production(A), LSV curves of different samples for electrochemical performance of NO oxidation and OER in 0.5 mol/L H2SO4(B), the Faradaic efficiencies of nitrate for the sample of P⁃CC(C), comparison of the electrochemical NO oxidation with OER(D) [ 70]
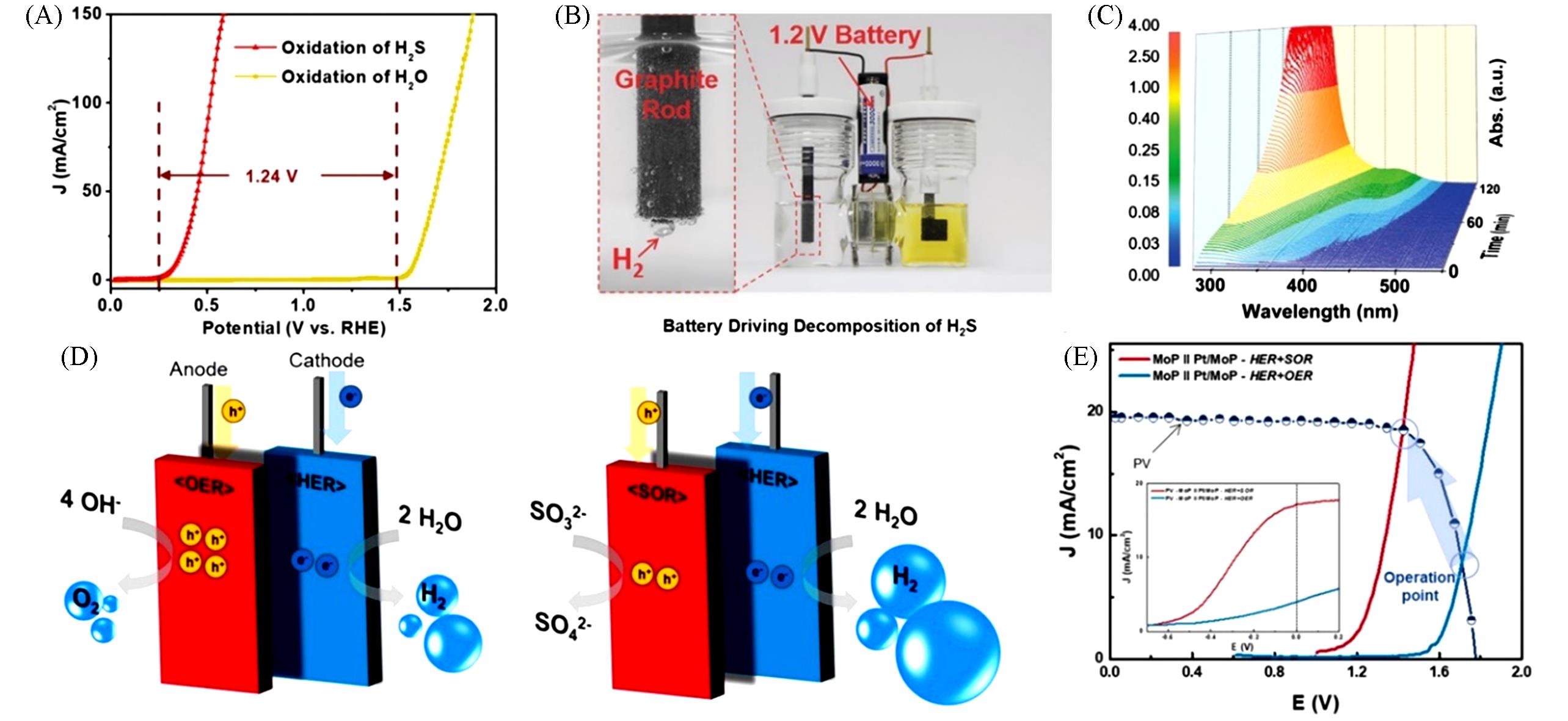
Fig.4 Comparison of SOR and OER polarization curves for CoNi@NGs(A), the photo of a device with a 1.2 V commercial battery directly driving the decomposition of H2S(B), in situ electrochemical UV⁃Vis tests for anodic electrolyte(C) [ 71], scheme for hydrogen production system using SOR instead of OER(D), J⁃ V curve of tandem photovoltaic cell powered electrolytic system for MoP||Pt/MoP catalysts(E) [ 74]
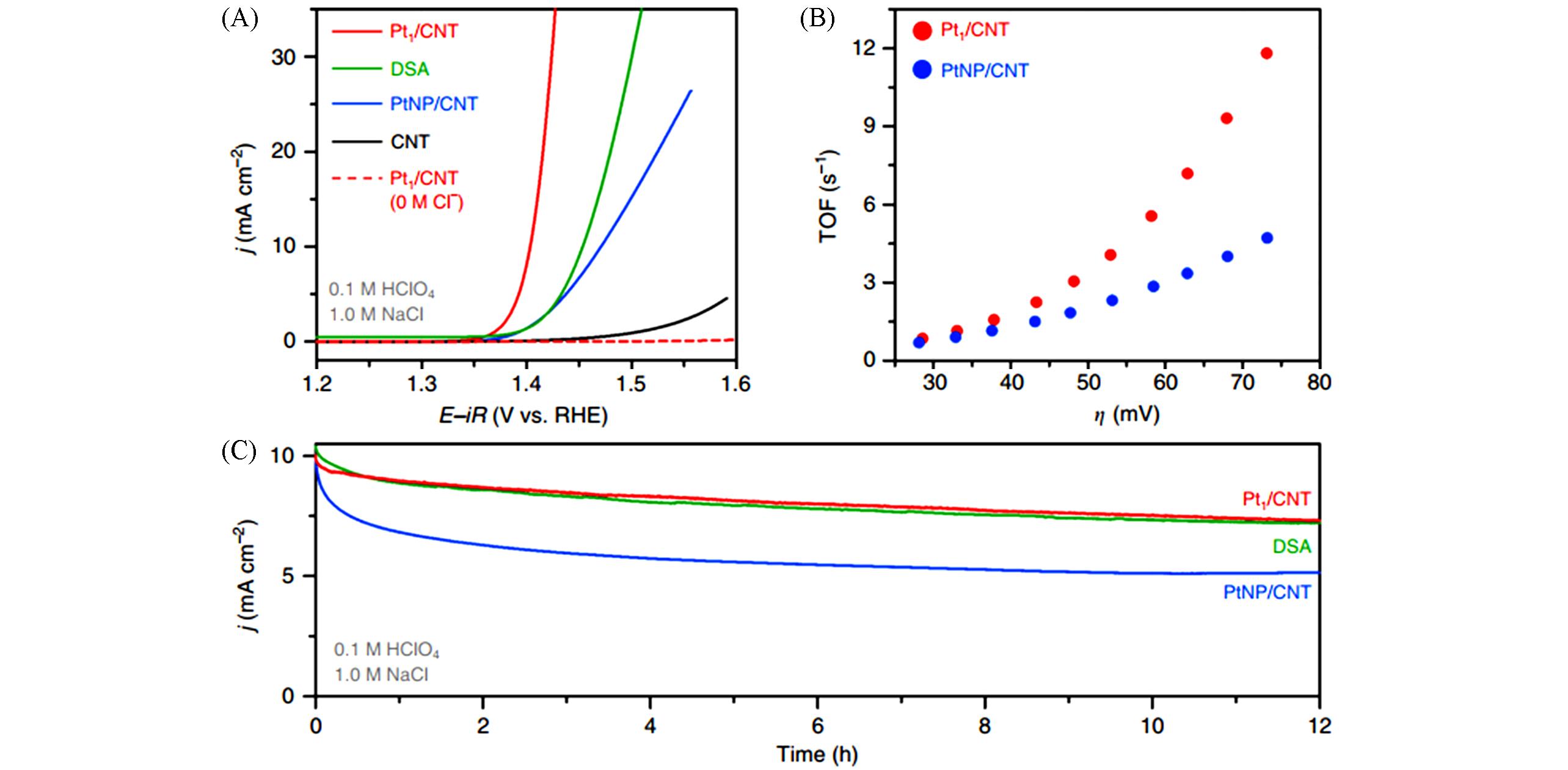
Fig.5 CER polarisation curves of different samples obtained in 0.1 mol/L HClO4+1.0 mol/L NaCl(A), calculated TOFs of Pt1/CNT and PtNP/CNT from the curves of (A)(B), chronoamperograms of Pt1/CNT and PtNP/CNT catalysts measured in 0.1 mol/L HClO4 + 1.0 mol/L NaCl for 12 h(C) [ 76]
| Inorganic chemical | Electrocatalyst | Performance( vs. RHE) | Electrolyte | Reference |
|---|---|---|---|---|
| N2H4 | NiCo/MXene | 43 mV at 500 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ |
| RuP2 | -70 mV at 10 mA/cm 2 | 1 mol/L KOH+0.3 mol/L N2H4 | [ | |
| Ni/C | -20 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| Co3N | -55 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| CoSe2 | -17 mV at 10 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ | |
| NO | Carbon cloth | 1.07 V at 10 mA/cm 2 | 0.5 mol/L H2SO4+ NO | [ |
| H2S | CoNi nanoalloy | 0.25 V at 1 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ |
| CoFeS2 | 0.6 V at 200 mA/cm 2 | H2S saturated 1 mol/L NaOH | [ | |
| Cu2S | 0.26 V at 10 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ | |
| Cl2 | Pt1/CNT | 1.4 V at 10 mA/cm 2 | 0.1 mol/L HClO4+ 1 mol/L of Cl - | [ |
| Co3O4 | 1.56 V at 10 mA/cm 2 | Saturated NaCl solution | [ |
Table 1 Summary of recent studies on electrochemical hydrogen production coupling with oxidation of inorganic chemicals
| Inorganic chemical | Electrocatalyst | Performance( vs. RHE) | Electrolyte | Reference |
|---|---|---|---|---|
| N2H4 | NiCo/MXene | 43 mV at 500 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ |
| RuP2 | -70 mV at 10 mA/cm 2 | 1 mol/L KOH+0.3 mol/L N2H4 | [ | |
| Ni/C | -20 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| Co3N | -55 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| CoSe2 | -17 mV at 10 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ | |
| NO | Carbon cloth | 1.07 V at 10 mA/cm 2 | 0.5 mol/L H2SO4+ NO | [ |
| H2S | CoNi nanoalloy | 0.25 V at 1 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ |
| CoFeS2 | 0.6 V at 200 mA/cm 2 | H2S saturated 1 mol/L NaOH | [ | |
| Cu2S | 0.26 V at 10 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ | |
| Cl2 | Pt1/CNT | 1.4 V at 10 mA/cm 2 | 0.1 mol/L HClO4+ 1 mol/L of Cl - | [ |
| Co3O4 | 1.56 V at 10 mA/cm 2 | Saturated NaCl solution | [ |
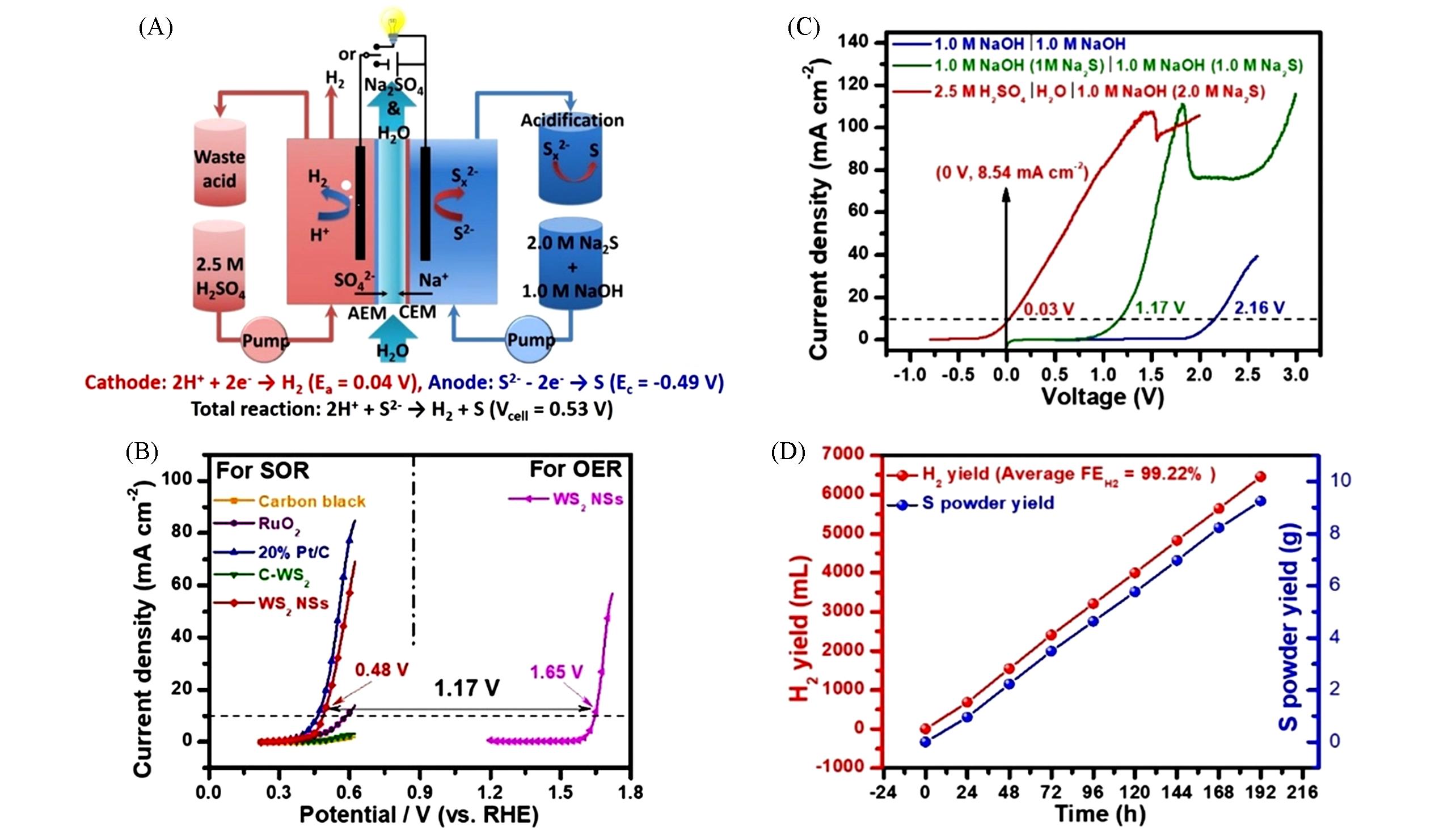
Fig.6 Illustration of the design of the H2 production system coupled with SOR and ENE(A), comparison of LSV curves for different system designs of SOR/HER or OER/HER cell(B), the polarization curves of different samples for electrochemical performance of SOR(C) and the results of yield rates of H2 and sulfur powder(D) [ 55]
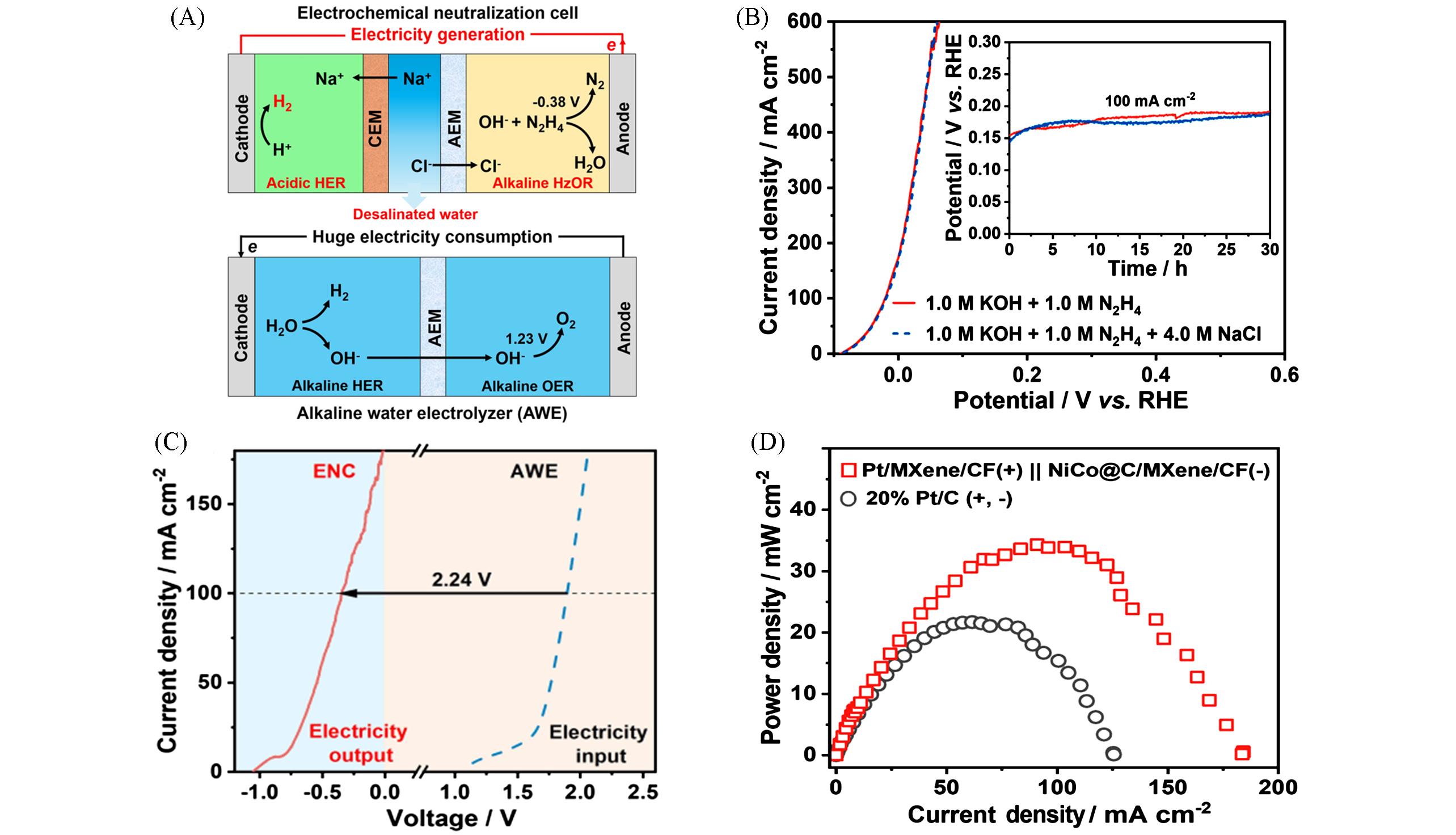
Fig.7 Illustration of the ENE cell for hydrogen production and water desalination with electricity output(A), the LSVs of the electrocatalyst for the HzOR with and without Cl - (B), a comparison of LSVs of electrochemical neutralization cell(ENC) and alkaline water electrolysis(AWE)(C) and the power⁃density curves of the ENC using different samples(D) [ 57](B) Inset are the chronopotentiometric curves at a current density of 100 mA/cm 2 with and without Cl -.Copyright 2022, Wiley-VCH.
| 1 | Turner J. A., Science, 2004, 305, 972—974 |
| 2 | Bogdanov D., Ram M., Aghahosseini A., Gulagi A., Oyewo A. S., Child M., Caldera U., Sadovskaia K., Farfan J., Barbosa L. D. S. N. S., Energy, 2021, 227, 120467 |
| 3 | Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., Chem. Soc. Rev., 2015, 44, 2060—2086 |
| 4 | Wang Z., Xiao B., Lin Z., Xu Y., Lin Y., Meng F., Zhang Q., Gu L., Fang B., Guo S., Zhong W., Angew. Chem. Int. Ed., 2021, 60, 23388—23393 |
| 5 | Yan D., Dou S., Tao L., Liu Z., Liu Z., Huo J., Wang S., J. Mater. Chem. A, 2016, 4, 13726—13730 |
| 6 | Li M., Zhao Z., Xia Z., Luo M., Zhang Q., Qin Y., Tao L., Yin K., Chao Y., Gu L., Yang W., Yu Y., Lu G., Guo S., Angew. Chem. Int. Ed., 2021, 60, 8243—8250 |
| 7 | Luo Y., Zhang Z., Chhowalla M., Liu B., Adv. Mater., 2022, 34, 2108133 |
| 8 | Xie X. Q., Liu J., Gu C., Li J., Zhao Y., Liu C. S., J. Energy Chem., 2022, 64, 503—510 |
| 9 | Wang Y., Yan D., El Hankari S., Zou Y., Wang S., Adv. Sci., 2018, 5, 1800064 |
| 10 | Kim J. S., Kim B., Kim H., Kang K., Adv. Energy Mater., 2018, 8, 1702774 |
| 11 | Ham K., Hong S., Kang S., Cho K., Lee J., ACS Energy Lett., 2021, 6, 364—370 |
| 12 | Roger I., Shipman M. A., Symes M. D., Nat. Rev. Chem., 2017, 1, 1—13 |
| 13 | Jia Y., Zhang L., Gao G., Chen H., Wang B., Zhou J., Soo M. T., Hong M., Yan X., Qian G., Adv. Mater., 2017, 29, 1700017 |
| 14 | Faber M. S., Jin S., Energy Environ. Sci., 2014, 7, 3519—3542 |
| 15 | Shen Z., Qu M., Shi J., Oropeza F. E., de la Peña O'Shea V. A., Gorni G., Tian C. M., Hofmann J. P., Cheng J., Li J., Zhang K. H. L., J. Energy Chem., 2022, 65, 637—645 |
| 16 | Yan D., Xia C., Zhang W., Hu Q., He C., Xia B. Y., Wang S., Adv. Energy Mater., 2022, 12, 2202317 |
| 17 | You B., Sun Y., Acc. Chem. Res., 2018, 51, 1571—1580 |
| 18 | Yu F., Yu L., Mishra I., Yu Y., Ren Z., Zhou H., Mater. Today Phys., 2018, 7, 121—138 |
| 19 | Xiao Y., Shen Y., Su D., Zhang S., Yang J., Yan D., Fang S., Wang X., J. Mater. Sci. Technol., 2023, 154, 1—8 |
| 20 | Li S., Chen B., Wang Y., Ye M. Y., van Aken P. A., Cheng C., Thomas A., Nat. Mater., 2021, 20, 1240—1247 |
| 21 | Yan D., Li Y., Huo J., Chen R., Dai L., Wang S., Adv. Mater., 2017, 29, 1606459 |
| 22 | Yan D., Li H., Chen C., Zou Y., Wang S., Small Methods, 2019, 3, 1800331 |
| 23 | Xie C., Yan D., Chen W., Zou Y., Chen R., Zang S., Wang Y., Yao X., Wang S., Mater. Today, 2019, 31, 47—68 |
| 24 | Li W., Zhao L., Jiang X., Chen Z., Zhang Y., Wang S., Adv. Funct. Mater., 2022, 2207727 |
| 25 | Xie L., Wang L., Zhao W., Liu S., Huang W., Zhao Q., Nat. Commun., 2021, 12, 5070 |
| 26 | Chen S., Li W. H., Jiang W., Yang J., Zhu J., Wang L., Ou H., Zhuang Z., Chen M., Sun X., Wang D., Li Y., Angew. Chem. Int. Ed., 2022, 61, e202114450 |
| 27 | Yan D., Chen R., Xiao Z., Wang S., Electrochim. Acta, 2019, 303, 316—322 |
| 28 | Yan D., Xia C., He C., Liu Q., Chen G., Guo W., Xia B. Y., Small, 2022, 18, 2106606 |
| 29 | Rausch B., Symes M. D., Chisholm G., Cronin L., Science, 2014, 345, 1326—1330 |
| 30 | Du L., Sun Y., You B., Mater. Rep. Energy, 2021, 1, 100004 |
| 31 | Symes M. D., Cronin L., Nat. Chem., 2013, 5, 403—409 |
| 32 | You B., Liu X., Liu X., Sun Y., ACS Catal., 2017, 7, 4564—4570 |
| 33 | You B., Jiang N., Liu X., Sun Y., Angew. Chem. Int. Ed., 2016, 55, 9913—9917 |
| 34 | Du J., Li F., Sun L., Chem. Soc. Rev., 2021, 50, 2663—2695 |
| 35 | Chen G., Li X., Feng X., Angew. Chem. Int. Ed., 2022, e202209014 |
| 36 | Wang T., Cao X., Jiao L., Angew. Chem. Int. Ed., 2022, e202213328 |
| 37 | Li R., Xiang K., Peng Z., Zou Y., Wang S., Adv. Energy Mater., 2021, 11, 2102292 |
| 38 | Zhang Y., Zhou B., Wei Z., Zhou W., Wang D., Tian J., Wang T., Zhao S., Liu J., Tao L., Wang S., Adv. Mater., 2021, 33, 2104791 |
| 39 | Chen Y., Lavacchi A., Miller H., Bevilacqua M., Filippi J., Innocenti M., Marchionni A., Oberhauser W., Wang L., Vizza F., Nat. Commun., 2014, 5, 4036 |
| 40 | Barwe S., Weidner J., Cychy S., Morales D. M., Dieckhöfer S., Hiltrop D., Masa J., Muhler M., Schuhmann W., Angew. Chem. Int. Ed., 2018, 57, 11460—11464 |
| 41 | Tong Y., Chen P., Zhang M., Zhou T., Zhang L., Chu W., Wu C., Xie Y., ACS Catal., 2018, 8, 1—7 |
| 42 | Huang Y., Chong X., Liu C., Liang Y., Zhang B., Angew. Chem., 2018, 130, 13347—13350 |
| 43 | Zhou B., Li Y., Zou Y., Chen W., Zhou W., Song M., Wu Y., Lu Y., Liu J., Wang Y., Wang S., Angew. Chem. Int. Ed., 2021, 60, 22908—22914 |
| 44 | Chen W., Xu L., Zhu X., Huang Y. C., Zhou W., Wang D., Zhou Y., Du S., Li Q., Xie C., Tao L., Dong C. L., Liu J., Wang Y., Chen R., Su H., Chen C., Zou Y., Li Y., Liu Q., Wang S., Angew. Chem. Int. Ed., 2021, 60, 7297—7307 |
| 45 | Wang T., Huang Z., Liu T., Tao L., Tian J., Gu K., Wei X., Zhou P., Gan L., Du S., Zou Y., Chen R., Li Y., Fu X. Z., Wang S., Angew. Chem. Int. Ed., 2022, 61, e202115636 |
| 46 | Deng C., Toe C. Y., Li X., Tan J., Yang H., Hu Q., He C., Adv. Energy Mater., 2022, 12, 2201047 |
| 47 | Cha H. G., Choi K. S., Nat. Chem., 2015, 7, 328—333 |
| 48 | Lhermitte C. R., Sivula K., ACS Catal., 2019, 9, 2007—2017 |
| 49 | Yan D., Mebrahtu C., Wang S., Palkovits R., Angew. Chem. Int. Ed., 2022. e202214333 |
| 50 | Chen L., Shi J., J. Mater. Chem. A, 2018, 6, 13538—13548 |
| 51 | Luo H., Barrio J., Sunny N., Li A., Steier L., Shah N., Stephens I. E., Titirici M. M., Adv. Energy Mater., 2021, 11, 2101180 |
| 52 | Ifkovits Z. P., Evans J. M., Meier M. C., Papadantonakis K. M., Lewis N. S., Energy Environ. Sci., 2021. 14, 4740—4759 |
| 53 | Li Y., Wei X., Chen L., Shi J., Angew. Chem. Int. Ed., 2021, 60, 19550—19571 |
| 54 | Anantharaj S., Noda S., Jothi V. R., Yi S., Driess M., Menezes P. W., Angew. Chem. Int. Ed., 2021, 60, 18981—19006 |
| 55 | Yi L., Ji Y., Shao P., Chen J., Li J., Li H., Chen K., Peng X., Wen Z., Angew. Chem. Int. Ed., 2021, 60, 21550—21557 |
| 56 | Wang D., He N., Xiao L., Dong F., Chen W., Zhou Y., Chen C., Wang S., Angew. Chem., 2021, 133, 24810—24816 |
| 57 | Sun F., He D., Yang K., Qiu J., Wang Z., Angew. Chem. Int. Ed., 2022, e202203929 |
| 58 | Zhang J. Y., Wang H., Tian Y., Yan Y., Xue Q., He T., Liu H., Wang C., Chen Y., Xia B. Y., Angew. Chem. Int. Ed., 2018, 57, 7649—7653 |
| 59 | Li Y., Zhang J., Liu Y., Qian Q., Li Z., Zhu Y., Zhang G., Sci. Adv., 2020, 6, eabb4197 |
| 60 | Liu X., He J., Zhao S., Liu Y., Zhao Z., Luo J., Hu G., Sun X., Ding Y., Nat. Commun., 2018, 9, 4365 |
| 61 | Sun F., Qin J., Wang Z., Yu M., Wu X., Sun X., Qiu J., Nat. Commun., 2021, 12, 4182 |
| 62 | Zhuang S., Tang Y., Tai X., Huang Q., Wan P., Chen Y., Sun Y., Pan J., Yang X. J., Appl. Catal. B: Environ., 2022, 306, 121132 |
| 63 | Liu Y., Zhang J., Li Y., Qian Q., Li Z., Zhu Y., Zhang G., Nat. Commun., 2020, 11, 1853 |
| 64 | Hu W., Selleri T., Gramigni F., Fenes E., Rout K. R., Liu S., Nova I., Chen D., Gao X., Tronconi E., Angew. Chem., 2021, 133, 7273—7280 |
| 65 | Zhang L., Liang J., Wang Y., Mou T., Lin Y., Yue L., Li T., Liu Q., Luo Y., Li N., Tang B., Liu Y., Gao S., Alshehri A. A., Guo X., Ma D., Sun X., Angew. Chem. Int. Ed., 2021, 60, 25263—25268 |
| 66 | Liu P., Liang J., Wang J., Zhang L., Li J., Yue L., Ren Y., Li T., Luo Y., Li N., Tang B., Liu Q., Asiri A. M., Kong Q., Sun X., Chem. Commun., 2021, 57, 13562—13565 |
| 67 | Ko B. H., Hasa B., Shin H., Zhao Y., Jiao F., J. Am. Chem. Soc., 2022, 144, 1258—1266 |
| 68 | Li Y., Cheng C., Han S., Huang Y., Du X., Zhang B., Yu Y., ACS Energy Lett., 2022, 7, 1187—1194 |
| 69 | Berisha L. S., Kalcher K., Maloku A., Andoni E., Arbneshi T., J. Adv. Chem., 2009, 5, 792—799 |
| 70 | Wang D., He N., Xiao L., Dong F., Chen W., Zhou Y., Chen C., Wang S., Angew. Chem. Int. Ed., 2021, 60, 24605—24611 |
| 71 | Zhang M., Guan J., Tu Y., Chen S., Wang Y., Wang S., Yu L., Ma C., Deng D., Bao X., Energy Environ. Sci., 2020, 13, 119—126 |
| 72 | Kumar M., Nagaiah T. C., J. Mater. Chem. A, 2022, 10, 7048—7057 |
| 73 | Pei Y., Cheng J., Zhong H., Pi Z., Zhao Y., Jin F., Green Chem., 2021, 23, 6975—6983 |
| 74 | Park J., Yoon H., Lee D. Y., Ji S. G., Yang W., Tilley S. D., Sung M. C., Park I. J., Tan J., Lee H., Kim J. Y., Kim D. W., Moon J., Appl. Catal. B: Environ., 2022, 305, 121045 |
| 75 | Zhu X., Wang P., Wang Z., Liu Y., Zheng Z., Zhang Q., Zhang X., Dai Y., Whangbo M. H., Huang B., J. Mater. Chem. A, 2018, 6, 12718—12723 |
| 76 | Lim T., Jung G. Y., Kim J. H., Park S. O., Park J., Kim Y. T., Kang S. J., Jeong H. Y., Kwak S. K., Joo S. H., Nat. Commun., 2020, 11, 412 |
| 77 | Zhu Y., Zhang J., Qian Q., Li Y., Li Z., Liu Y., Xiao C., Zhang G., Xie Y., Angew. Chem. Int. Ed., 2022, 61, e202113082 |
| 78 | Ding Y., Cai P., Wen Z., Chem. Soc. Rev., 2021, 50, 1495—1511 |
| 79 | Zhang M., Chen J., Li H., Cai P., Li Y., Wen Z., Nano Energy, 2019, 61, 576—583 |
| 80 | Bhat Z. M., Pandit D., Ardo S., Thimmappa R., Kottaichamy A. R., Dargily N. C., Devendrachari M. C., Thotiyl M. O., Joule, 2020, 4, 1730—1742 |
| 81 | Zhang M., Li H., Cai P., Chen K., Wen Z., Adv. Funct. Mater., 2021, 31, 2103248 |
| 82 | Wang G., Chen J., Cai P., Jia J., Wen Z., J. Mater. Chem. A, 2018, 6, 17763—17770 |
| [1] | 吕家根. 一种新的与微型化自发电池整合的电化学发光检测芯片研究[J]. 高等学校化学学报, 2004, 25(S1): 104. |
| [2] | 刘庆林, 李鹏, 张志炳. 精馏节能过程非平衡热力学分析——模型方程的建立[J]. 高等学校化学学报, 2001, 22(7): 1209. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||