

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (4): 725.doi: 10.7503/cjcu20180599
张硕( ), 于一涛, 李庆刚, 赵宁, 侯梓桐, 刘一帆, 李冰, 牟秋红, 李金辉, 王峰, 彭丹(
), 于一涛, 李庆刚, 赵宁, 侯梓桐, 刘一帆, 李冰, 牟秋红, 李金辉, 王峰, 彭丹( )
)
收稿日期:2018-08-27
出版日期:2019-04-03
发布日期:2019-01-15
作者简介:联系人简介: 张 硕, 男, 博士, 助理研究员, 主要从事有机催化反应方面的研究. E-mail:
基金资助:
ZHANG Shuo*( ), YU Yitao, LI Qinggang, ZHAO Ning, HOU Zitong, LIU Yifan, LI Bing, MU Qiuhong, LI Jinhui, WANG Feng, PENG Dan*(
), YU Yitao, LI Qinggang, ZHAO Ning, HOU Zitong, LIU Yifan, LI Bing, MU Qiuhong, LI Jinhui, WANG Feng, PENG Dan*( )
)
Received:2018-08-27
Online:2019-04-03
Published:2019-01-15
Contact:
ZHANG Shuo,PENG Dan
E-mail:e50687e@163.com;lonarpeng@aliyun.com
Supported by:摘要:
以2-[羟基(苯基)甲基]苯酚类化合物和简单的硫醇为原料, 1,2-二氯乙烷为溶剂, 在Sc(Ⅲ)促进下原位生成邻亚甲基苯醌, 并发生亲核加成反应构建邻羟基苄硫醚. 该反应在50 ℃下搅拌2 h即可完成, 目标产物产率82%95%. 反应可放大至克级规模.
中图分类号:
TrendMD:
张硕, 于一涛, 李庆刚, 赵宁, 侯梓桐, 刘一帆, 李冰, 牟秋红, 李金辉, 王峰, 彭丹. Sc(Ⅲ)催化硫醇对邻亚甲基苯醌的亲核加成反应合成邻羟基苄硫醚衍生物. 高等学校化学学报, 2019, 40(4): 725.
ZHANG Shuo,YU Yitao,LI Qinggang,ZHAO Ning,HOU Zitong,LIU Yifan,LI Bing,MU Qiuhong,LI Jinhui,WANG Feng,PENG Dan. Sc(Ⅲ) Catalyzed Nucleophilic Addition of in situ Generated ortho-Quinone Methides with Thiols: an Efficient Access to ortho-Hydroxybenzyl Thioethers†. Chem. J. Chinese Universities, 2019, 40(4): 725.
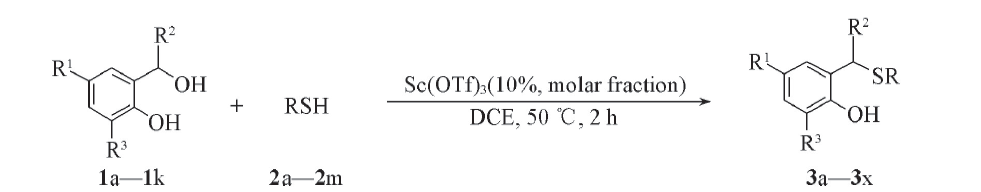
Scheme 1 Sc(OTf)3 catalyzed nucleophilic addition reaction by thiols3a: R1=H, R2=Ph, R3=H, R=C2H5; 3b: R1=H, R2=Ph, R3=H, R=n-propyl; 3c: R1=H, R2=Ph, R3=H, R=n-pentyl; 3e: R1=H, R2=Ph, R3=H, R=heptyl; 3f: R1=H, R2=Ph, R3=H, R=isobutyl; 3g: R1=H, R2=Ph, R3=H, R=isopentyl; 3h: R1=H, R2=Ph, R3=H, R=isopropyl; 3i: R1=H, R2=Ph, R3=H, R=cyclopentyl; 3j: R1=H, R2=Ph, R3=H, R=benzyl; 3k: R1=H, R2=Ph, R3=H, R=4-(tert-butyl)benzyl; 3l: R1=H, R2=Ph, R3=H, R=4-chlorobenzyl; 3m: R1=H, R2=Ph, R3=H, R=4-methylbenzyl; 3n: R1=H, R2=Ph, R3=tert-butyl, R=C2H5; 3o: R1=H, R2=Ph, R3=ethoxy, R=C2H5; 3p: R1=CH3, R2=Ph, R3=H, R=C2H5; 3q: R1=emthoxy, R2=Ph, R3=H, R=C2H5; 3r: R1=H, R2=o-tolyl, R3=H, R=C2H5; 3s: R1=H, R2=4-chlorophenyl, R3=H, R=C2H5; 3t: R1=H, R2=3-fluorophenyl, R3=H, R=C2H5; 3u: R1=H, R2=4-fluorophenyl, R3=H, R=C2H5; 3v: R1=Cl, R2=Ph, R3=H, R=C2H5; 3w: R1=H, R2=cyclohexyl, R3=H, R=C2H5; 3x: R1=H, R2=H, R3=H, R=C2H5
| Entry | Catalyst | Solvent | Temperature/℃ | t/h | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | Ga(OTf)3 | DCE | 50 | 4 | 0 |
| 2 | In(OTf)3 | DCE | 50 | 4 | 0 |
| 3 | Sc(OTf)3 | DCE | 50 | 4 | 92 |
| 4 | Conc. HCl | DCE | 50 | 8 | 56 |
| 5 | H2SO4 | DCE | 50 | 8 | 52 |
| 6 | AlCl3 | DCE | 50 | 8 | 62 |
| 7 | Sc(OTf)3 | Toluene | 50 | 4 | 72 |
| 8 | Sc(OTf)3 | THF | 50 | 4 | 76 |
| 9 | Sc(OTf)3 | DCE | 50 | 2 | 92 |
| 10 | Sc(OTf)3 | DCE | 50 | 1 | 75 |
| 11 | Sc(OTf)3 | DCE | 40 | 6 | 82 |
| 12c | Sc(OTf)3 | DCE | 50 | 6 | 78 |
| 13d | Sc(OTf)3 | DCE | 50 | 2 | 93 |
Table 1 Optimization of reaction conditionsa
| Entry | Catalyst | Solvent | Temperature/℃ | t/h | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | Ga(OTf)3 | DCE | 50 | 4 | 0 |
| 2 | In(OTf)3 | DCE | 50 | 4 | 0 |
| 3 | Sc(OTf)3 | DCE | 50 | 4 | 92 |
| 4 | Conc. HCl | DCE | 50 | 8 | 56 |
| 5 | H2SO4 | DCE | 50 | 8 | 52 |
| 6 | AlCl3 | DCE | 50 | 8 | 62 |
| 7 | Sc(OTf)3 | Toluene | 50 | 4 | 72 |
| 8 | Sc(OTf)3 | THF | 50 | 4 | 76 |
| 9 | Sc(OTf)3 | DCE | 50 | 2 | 92 |
| 10 | Sc(OTf)3 | DCE | 50 | 1 | 75 |
| 11 | Sc(OTf)3 | DCE | 40 | 6 | 82 |
| 12c | Sc(OTf)3 | DCE | 50 | 6 | 78 |
| 13d | Sc(OTf)3 | DCE | 50 | 2 | 93 |
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
|---|---|---|---|---|
| 3a | Colorless oil | 92 | 245.0996 | 3290, 3263, 3029, 1596, 1582, 1488, 1454, 1340, 1269, 1234, 754, 699 |
| 3b | Colorless oil | 90 | 259.1150 | 3289, 3062, 3029, 1596, 1582, 1487, 1454, 1339, 1270, 1236, 754, 699 |
| 3c | Colorless oil | 86 | 273.1312 | 3291, 3029, 1596, 1582, 1487, 1454, 1356, 1272, 1233, 754, 699 |
| 3d | Colorless oil | 84 | 287.1465 | 3292, 3029, 1596, 1582, 1487, 1454, 1341, 1271, 1234, 754, 699 |
| 3e | Colorless oil | 82 | 315.1779 | 3290, 3029, 1596, 1582, 1487, 1454, 1356, 1271, 1234, 753, 699 |
| 3f | Colorless oil | 87 | 273.1305 | 3291, 3029, 1597, 1583, 1488, 1454, 1366, 1271, 1234, 754, 699 |
| 3g | Colorless oil | 85 | 287.1467 | 3290, 3029, 1597, 1583, 1488, 1454, 1367, 1273, 1233, 754, 699 |
| 3h[ | Colorless oil | 88 | 259.1150 | 3290, 3029, 1598, 1583, 1489, 1454, 1366, 1271, 1235, 754, 699 |
| 3i | Colorless oil | 86 | 285.1308 | 3289, 3028, 1644, 1597, 1489, 1453, 1365, 1271, 1235, 754, 699 |
| 3j[ | Colorless oil | 91 | 307.1152 | 3289, 3028, 1644, 1599, 1493, 1454, 1341, 1272, 1234, 755, 699 |
| 3k | Colorless oil | 95 | 363.1777 | 3289, 3030, 1647, 1599, 1488, 1456, 1365, 1271, 1236, 756, 701 |
| 3l | Colorless oil | 86 | 341.0762 | 3296, 3029, 1644, 1597, 1490, 1454, 1365, 1272, 1233, 755, 699 |
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
| 3m | Colorless oil | 92 | 321.1305 | 3288, 3027, 1645, 1596, 1486, 1454, 1353, 1271, 1235, 754, 699 |
| 3n | Colorless oil | 90 | 301.1622 | 3246, 3028, 1655, 1600, 1586, 1494, 1483, 1363, 1268, 1231, 749, 701 |
| 3o | Colorless oil | 82 | 289.1257 | 3525, 3028, 1662, 1614, 1598, 1471, 1452, 1372, 1271, 1220, 732, 698 |
| 3p | Colorless oil | 95 | 259.1151 | 3291, 3029, 1647, 1613, 1601, 1506, 1453, 1265, 1247, 702 |
| 3q | Colorless oil | 90 | 275.1102 | 3392, 3028, 1501, 1451, 1266, 1240, 701 |
| 3r | Colorless oil | 88 | 259.1151 | 3287, 3020, 1644, 1601, 1485, 1269, 1240, 753 |
| 3s | Colorless oil | 86 | 279.0604 | 3298, 2968, 2928, 1644, 1596, 1488, 1455, 1268, 1233, 755 |
| 3t | Colorless oil | 84 | 263.0901 | 3305, 2969, 2928, 1643, 1613, 1589, 1486, 1267, 1234, 755 |
| 3u | Colorless oil | 82 | 263.0903 | 3305, 2969, 2928, 1603, 1506, 1487, 1269, 1226, 755 |
| 3v | Colorless oil | 90 | 279.0605 | 3294, 2969, 2928, 1642, 1599, 1482, 1416, 1268, 1234, 698 |
| 3w | Colorless oil | 85 | 251.1464 | 3271, 2927, 2852, 1609, 1581, 1487, 1451, 1271, 1231, 754 |
| 3x[ | Colorless oil | 88 | 169.0685 | 269, 2968, 2926, 1612, 1597, 1450, 1265, 1226, 699 |
| 4a[ | Colorless oil | 85 | 245.0997 |
Table 2 Appearance, yields, melting points, HRMS and IR data for compounds 3a—3x and 4a
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
|---|---|---|---|---|
| 3a | Colorless oil | 92 | 245.0996 | 3290, 3263, 3029, 1596, 1582, 1488, 1454, 1340, 1269, 1234, 754, 699 |
| 3b | Colorless oil | 90 | 259.1150 | 3289, 3062, 3029, 1596, 1582, 1487, 1454, 1339, 1270, 1236, 754, 699 |
| 3c | Colorless oil | 86 | 273.1312 | 3291, 3029, 1596, 1582, 1487, 1454, 1356, 1272, 1233, 754, 699 |
| 3d | Colorless oil | 84 | 287.1465 | 3292, 3029, 1596, 1582, 1487, 1454, 1341, 1271, 1234, 754, 699 |
| 3e | Colorless oil | 82 | 315.1779 | 3290, 3029, 1596, 1582, 1487, 1454, 1356, 1271, 1234, 753, 699 |
| 3f | Colorless oil | 87 | 273.1305 | 3291, 3029, 1597, 1583, 1488, 1454, 1366, 1271, 1234, 754, 699 |
| 3g | Colorless oil | 85 | 287.1467 | 3290, 3029, 1597, 1583, 1488, 1454, 1367, 1273, 1233, 754, 699 |
| 3h[ | Colorless oil | 88 | 259.1150 | 3290, 3029, 1598, 1583, 1489, 1454, 1366, 1271, 1235, 754, 699 |
| 3i | Colorless oil | 86 | 285.1308 | 3289, 3028, 1644, 1597, 1489, 1453, 1365, 1271, 1235, 754, 699 |
| 3j[ | Colorless oil | 91 | 307.1152 | 3289, 3028, 1644, 1599, 1493, 1454, 1341, 1272, 1234, 755, 699 |
| 3k | Colorless oil | 95 | 363.1777 | 3289, 3030, 1647, 1599, 1488, 1456, 1365, 1271, 1236, 756, 701 |
| 3l | Colorless oil | 86 | 341.0762 | 3296, 3029, 1644, 1597, 1490, 1454, 1365, 1272, 1233, 755, 699 |
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
| 3m | Colorless oil | 92 | 321.1305 | 3288, 3027, 1645, 1596, 1486, 1454, 1353, 1271, 1235, 754, 699 |
| 3n | Colorless oil | 90 | 301.1622 | 3246, 3028, 1655, 1600, 1586, 1494, 1483, 1363, 1268, 1231, 749, 701 |
| 3o | Colorless oil | 82 | 289.1257 | 3525, 3028, 1662, 1614, 1598, 1471, 1452, 1372, 1271, 1220, 732, 698 |
| 3p | Colorless oil | 95 | 259.1151 | 3291, 3029, 1647, 1613, 1601, 1506, 1453, 1265, 1247, 702 |
| 3q | Colorless oil | 90 | 275.1102 | 3392, 3028, 1501, 1451, 1266, 1240, 701 |
| 3r | Colorless oil | 88 | 259.1151 | 3287, 3020, 1644, 1601, 1485, 1269, 1240, 753 |
| 3s | Colorless oil | 86 | 279.0604 | 3298, 2968, 2928, 1644, 1596, 1488, 1455, 1268, 1233, 755 |
| 3t | Colorless oil | 84 | 263.0901 | 3305, 2969, 2928, 1643, 1613, 1589, 1486, 1267, 1234, 755 |
| 3u | Colorless oil | 82 | 263.0903 | 3305, 2969, 2928, 1603, 1506, 1487, 1269, 1226, 755 |
| 3v | Colorless oil | 90 | 279.0605 | 3294, 2969, 2928, 1642, 1599, 1482, 1416, 1268, 1234, 698 |
| 3w | Colorless oil | 85 | 251.1464 | 3271, 2927, 2852, 1609, 1581, 1487, 1451, 1271, 1231, 754 |
| 3x[ | Colorless oil | 88 | 169.0685 | 269, 2968, 2926, 1612, 1597, 1450, 1265, 1226, 699 |
| 4a[ | Colorless oil | 85 | 245.0997 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
|---|---|---|
| 3a | 7.40(d, J=7.4 Hz, 2H), 7.32—7.16(m, 5H), 7.05(dd, J=7.6, 1.2 Hz, 1H), 6.91(dd, J=8.0, 0.7 Hz, 1H), 6.88—6.82(m, 1H), 5.39(s, 1H), 2.49—2.43(m, 2H), 1.23(t, J=7.4 Hz, 3H) | 155.15, 139.31, 130.17, 129.21, 128.69, 128.67, 128.53, 128.51, 127.55, 125.16, 120.76, 117.60, 50.47, 26.36, 14.20 |
| 3b | 7.40(d, J=7.4 Hz, 2H), 7.34—7.29(m, 2H), 7.21(ddd, J=15.4, 10.6, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.95—6.89(m, 1H), 6.84(td, J=7.6, 1.0 Hz, 1H), 5.35(s, 1H), 2.48—2.39(m, 2H), 1.61(dd, J=14.6, 7.3 Hz, 2H), 0.95(t, J=7.4 Hz, 3H) | 155.22, 139.30, 130.18, 129.21, 128.66, 128.48, 127.53, 125.11, 120.70, 117.64, 50.97, 34.33, 22.32, 13.49 |
| 3c | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=10.0, 4.8 Hz, 3H), 7.26—7.11(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.90(m, 1H), 6.88—6.82(m, 1H), 5.35(s, 1H), 2.49—2.42(m, 2H), 1.63—1.51(m, 2H), 1.38—1.33(m, 2H), 0.85(t, J=7.3 Hz, 3H) | 155.23, 139.29, 130.19, 129.21, 128.67, 128.51, 128.49, 128.46, 127.54, 125.11, 120.71, 117.64, 51.04, 32.02, 21.01, 21.96, 13.64 |
| 3d | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=9.7, 5.1 Hz, 3H), 7.28—7.13(m, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.96—6.88(m, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.45(tq, J=10.7, 5.3 Hz, 2H), 1.57(dd, J=14.7, 7.3 Hz, 2H), 1.31—1.26(m, 4H), 0.85(t, J=7.1 Hz, 3H) | 155.23, 139.31, 130.19, 129.21, 128.67, 128.51, 128.48, 128.46, 127.53, 125.12, 120.71, 117.63, 51.05, 32.33, 30.99, 28.61, 22.23, 13.94 |
| 3e | 7.40(d, J=7.5 Hz, 2H), 7.31(dd, J=9.9, 4.9 Hz, 3H), 7.27—7.17(m, 2H), 7.03(dd, J=7.6, 1.2 Hz, 1H), 6.92(dd, J=8.0, 0.6 Hz, 1H), 6.86—6.82(m, 1H), 5.34(s, 1H), 2.45(tq, J=10.8, 5.3 Hz, 2H), 1.60—1.56(m, 2H), 1.32—1.23(m, 8H), 0.86(t, J=6.9 Hz, 3H) | 155.25, 139.29, 130.18, 129.21, 128.66, 128.54, 128.49, 127.53, 125.09, 120.70, 117.65, 51.08, 32.34, 31.67, 28.92, 28.80, 28.77, 22.61, 14.09 |
| 3f | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=13.8, 6.6 Hz, 3H), 7.21(ddd, J=11.3, 8.7, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.92(dd, J= 8.0, 0.6 Hz, 1H), 6.87—6.80(m, 1H), 5.31(s, 1H), 2.35(qd, J=12.5, 6.8 Hz, 2H), 1.83(dt, J=13.4, 6.7 Hz, 1H), 0.95(dd, J=8.0, 6.8 Hz, 6H) | 155.26, 139.36, 130.24, 129.22, 128.66, 128.51, 128.48, 128.45, 127.54, 125.12, 120.69, 117.65, 51.54, 41.27, 28.24, 22.22, 21.98 |
| 3g | 7.40(d, J=7.4 Hz, 2H), 7.36—7.26(m, 3H), 7.21(ddd, J=17.1, 11.2, 4.3 Hz, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.92(d, J=7.7 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.48—2.43(m, 2H), 1.61(dt, J=13.3, 6.6 Hz, 1H), 1.50—1.45(m, 2H), 0.84—0.81(m, 6H) | 155.22, 139.26, 130.19, 129.23, 128.66, 128.51, 128.49, 128.46, 127.54, 125.06, 120.71, 117.64, 51.03, 37.92, 30.36, 27.40, 22.32, 22.15 |
| 3h | 7.49—7.34(m, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.14(m, 2H), 7.04(dd, J=7.6, 1.2 Hz, 1H), 6.92(d, J=8.0 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.41(s, 1H), 2.77(dt, J=13.4, 6.7 Hz, 1H), 1.29—1.24(m, 6H) | 155.30, 139.28, 130.09, 129.19, 128.67, 128.48, 127.50, 125.11, 120.73, 117.73, 50.13, 35.76, 23.07, 22.85 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3i | 7.40(d, J=7.3 Hz, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.13(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.88(m, 1H), 6.85(dd, J=10.8, 4.1 Hz, 1H), 5.36(s, 1H), 2.91(p, J=7.0 Hz, 1H), 1.91(dt, J=12.3, 6.3 Hz, 2H), 1.72—1.70(m, 2H), 1.64—1.50(m, 4H) | 155.33, 139.37, 130.05, 129.15, 128.64, 128.46, 128.44, 127.47, 125.36, 120.70, 117.71, 51.40, 44.23, 33.65, 33.20, 24.88, 24.74 |
| 3j | 7.36(d, J=7.3 Hz, 2H), 7.30(t, J=7.3 Hz, 4H), 7.27—7.22(m, 2H), 7.18(dd, J=12.7, 4.5 Hz, 3H), 6.95(dd, J=7.7, 1.3 Hz, 1H), 6.90(d, J= 8.4 Hz, 2H), 6.86—6.79(m, 1H), 5.11(s, 1H), 3.59(d, J=3.2 Hz, 2H) | 155.03, 138.75, 137.28, 130.31 , 129.30, 129.01, 128.99, 128.76, 128.73, 127.65, 127.47, 124.86, 120.80, 117.60, 49.68, 36.54 |
| 3k | 7.34(dt, J=14.9, 7.8 Hz, 6H), 7.27—7.19(m, 2H), 7.15(d, J=8.2 Hz, 2H), 6.99(s, 1H), 6.96—6.90(m, 2H), 6.88—6.79(m, 1H), 5.14(s, 1H), 3.59(d, J=2.5 Hz, 2H), 1.32(s, 9H) | 155.14, 150.39, 138.73, 134.04, 130.35, 129.27, 128.73, 128.67, 128.64, 127.59, 125.59, 124.78, 120.70, 117.64, 49.97, 36.13, 34.57, 31.37 |
| 3l | 7.36—7.18(m, 8H), 7.11(d, J=8.4 Hz, 2H), 6.99(dd, J=7.6, 1.3 Hz, 1H), 6.93—6.81(m, 2H), 6.78(s, 1H), 5.12(s, 1H), 3.56(s, 2H) | 154.87, 138.61, 135.81, 133.18, 130.32, 130.29, 129.35,128.81, 128.75, 128.64, 127.70, 124.63, 120.88, 117.52, 49.65, 35.89 |
| 3m | 7.40—7.29(m, 4H), 7.26—7.17(m, 2H), 7.13—7.08(m, 4H), 6.96(s, 1H), 6.93—6.90(m, 2H), 6.83(t, J=7.4 Hz, 1H), 5.09(s, 1H), 3.56(d, J=5.1 Hz, 2H), 2.33(s, 3H) | 155.08, 138.74, 137.14, 134.11, 130.31, 129.41, 129.26 , 128.90, 128.79, 128.71, 127.61, 124.87, 120.74, 117.62, 49.64, 36.21, 21.20 |
| 3n | 7.61(s, 1H), 7.42(d, J=7.4 Hz, 2H), 7.34(t, J=7.4 Hz, 2H), 7.30—7.21(m, 2H), 6.74(dd, J=7.2, 5.3 Hz, 2H), 5.35(s, 1H), 2.46(q, J=7.4 Hz, 2H), 1.44(s, 9H), 1.25(t, J=7.3 Hz, 3H) | 154.38, 138.88, 138.29, 128.82, 128.63, 128.04, 127.56, 126.66, 125.19, 119.80, 77.36, 77.05, 76.73, 51.54, 34.93, 29.76, 26.27, 14.24 |
| 3o | 7.47(d, J=7.4 Hz, 2H), 7.28(t, J=7.5 Hz, 2H), 7.22—7.08(m, 2H), 6.81(t, J=8.0 Hz, 1H), 6.71(dd, J=8.0, 1.1 Hz, 1H), 5.98(s, 1H), 5.66(s, 1H), 4.10—4.02(m, 2H), 2.44(q, J=7.4 Hz, 2H), 1.41(t, J=7.0 Hz, 3H), 1.23(t, J=7.4 Hz, 3H) | 145.71, 143.18, 141.48, 128.38, 128.35, 127.33, 126.88, 121.02, 119.63, 110.09, 64.57, 46.17, 26.31, 14.92, 14.31 |
| 3p | 7.40(d, J=7.5 Hz, 2H), 7.31(t, J=7.4 Hz, 2H), 7.26—7.22(m, 1H), 7.12(s, 1H), 7.00(dd, J=8.2, 1.7 Hz, 1H), 6.83(dd, J=8.1, 4.9 Hz, 2H), 5.34(s, 1H), 2.48(dd, J=7.4, 4.0 Hz, 2H), 2.21(s, 3H), 1.25(t, J=7.4 Hz, 3H) | 152.92, 139.33, 130.56, 129.85, 129.72, 128.65, 128.45, 127.48, 124.56, 117.49, 50.76, 26.36, 20.57, 14.16 |
| 3q | 7.41(d, J=7.4 Hz, 2H), 7.32(dd, J=10.1, 4.7 Hz, 2H), 7.25(t, J=7.2 Hz, 1H), 6.86(d, J=8.8 Hz, 1H), 6.76(dd, J=8.8, 3.1 Hz, 2H), 6.63(d, J=3.0 Hz, 1H), 5.34(s, 1H), 3.70(s, 3H), 2.48(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 153.54, 148.85, 139.05, 128.66, 128.64, 128.49, 128.44, 127.56, 126.25, 118.26, 115.74, 113.95, 55.66, 50.59, 26.35, 14.18 |
| 3r | 7.54—7.51(m, 1H), 7.29(s, 1H), 7.23—7.11(m, 4H), 6.94(ddd, J=11.9, 7.9, 1.0 Hz, 2H), 6.83—6.80(m, 1H), 5.52(s, 1H), 2.51(q, J=7.4 Hz, 2H), 2.39(s, 3H), 1.27(t, J=7.4 Hz, 3H) | 155.18, 137.18, 136.28, 130.73, 129.94, 128.98, 128.62, 127.48, 126.47, 125.15, 120.79, 117.44, 46.90, 26.47, 19.31, 14.16 |
| 3s | 7.34(d, J=8.5 Hz, 2H), 7.22(ddd, J=11.3, 8.7 Hz, 4.7 Hz, 2H), 7.08—7.03(m, 2H), 6.93—6.85(m, 2H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 154.95, 137.91, 133.29, 129.97, 129.84, 129.35, 128.78, 124.75, 120.86, 117.63, 49.68, 26.37, 14.12 |
| 3t | 7.30—7.26(m, 1H), 7.21(ddd, J=12.9, 9.8, 4.7 Hz, 2H), 7.14(dd, J=10.1, 2.0 Hz, 1H), 7.06(dd, J=7.6, 1.3 Hz, 1H), 7.01(s, 1H), 6.97—6.86(m, 3H), 5.38(s, 1H), 2.49(qd, J=7.4, 1.6 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 164.17, 161.72, 154.88, 142.07(d, J=6.9 Hz), 130.07(d, J=8.3 Hz), 129.99, 129.37, 124.76, 124.12(d, J=2.8 Hz), 120.89, 117.58, 115.63, 115.41, 114.56, 114.35, 49.70, 26.36, 14.12 |
| 3u | 7.39—7.35(m, 2H), 7.22(td, J=8.1, 1.6 Hz, 1H), 7.17(s, 1H), 7.05—6.98(m, 3H), 6.93(dd, J =8.0, 0.8 Hz, 1H), 6.87(td, J=7.5, 1.0 Hz, 1H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.7 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 162.04(d, J=245 Hz), 155.05, 134.99(d, J=3 Hz), 130.12, 130.03(d, J=2.6 Hz), 129.32, 124.87, 120.80, 117.69, 115.59, 115.38, 49.78, 26.35, 14.10 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3v | 7.40—7.25(m, 6H), 7.16(dd, J=8.6, 2.6 Hz, 1H), 7.03(d, J=2.5 Hz, 1H), 6.86(d, J=8.6 Hz, 1H), 5.31(s, 1H), 2.49(dd, J=13.7, 7.0 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 153.82, 138.45, 129.70, 129.03, 128.80, 128.76, 128.41, 128.36, 128.32, 127.82, 126.81, 125.44, 119.00, 50.32, 29.71, 26.41, 14.10 |
| 3w | 7.64(s, 1H), 7.18—7.14(m, 1H), 6.96(dd, J=7.5, 1.3 Hz, 1H), 6.86(d, J=7.7, 1H), 6.80(dd, J=11.5, 4.1 Hz, 1H), 3.78(d, J=9.1 Hz, 1H), 2.29(qd, J=7.4, 1.5 Hz, 2H), 2.14(d, J=12.8 Hz, 1H), 1.87—1.85(m, 1H), 1.74(dd, J=10.4, 2.7 Hz, 1H), 1.63—1.60(m, 2H), 1.41(d, J=13.0 Hz, 1H), 1.23(ddd, J=15.7, 8.6, 5.2 Hz, 1H), 1.15—1.03(m, 6H), 0.92—0.90(m, 1H) | 155.51, 131.14, 128.68, 124.72, 119.88, 117.59, 55.16, 41.45, 32.07, 32.02, 26.28, 26.16, 14.24 |
| 3x | 7.20(t, J=7.5 Hz, 1H), 7.08(d, J=7.2 Hz, 1H), 6.97—6.79(m, 2H), 6.75(s, 1H), 3.82(s, 2H), 2.42(q, J=7.3 Hz, 2H), 1.26—1.21(m, 3H) | 155.51, 130.45, 129.11, 122.44, 120.52, 117.20, 32.38 , 24.76, 14.23 |
| 4a | 7.40(d, J=7.4 Hz, 2H), 7.25(td, J=17.2, 7.3 Hz, 5H), 6.74(d, J=8.5 Hz, 2H), 5.79(s, 1H), 5.12(s, 1H), 2.37(q, J=7.4 Hz, 2H), 1.19(t, J=7.4 Hz, 3H) | 154.72, 141.81, 133.61, 129.56, 128.56, 128.26, 127.09, 115.49, 53.17, 26.27, 14.25 |
Table 3 1H NMR and 13C NMR data for compounds 3a—3x and 4a
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
|---|---|---|
| 3a | 7.40(d, J=7.4 Hz, 2H), 7.32—7.16(m, 5H), 7.05(dd, J=7.6, 1.2 Hz, 1H), 6.91(dd, J=8.0, 0.7 Hz, 1H), 6.88—6.82(m, 1H), 5.39(s, 1H), 2.49—2.43(m, 2H), 1.23(t, J=7.4 Hz, 3H) | 155.15, 139.31, 130.17, 129.21, 128.69, 128.67, 128.53, 128.51, 127.55, 125.16, 120.76, 117.60, 50.47, 26.36, 14.20 |
| 3b | 7.40(d, J=7.4 Hz, 2H), 7.34—7.29(m, 2H), 7.21(ddd, J=15.4, 10.6, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.95—6.89(m, 1H), 6.84(td, J=7.6, 1.0 Hz, 1H), 5.35(s, 1H), 2.48—2.39(m, 2H), 1.61(dd, J=14.6, 7.3 Hz, 2H), 0.95(t, J=7.4 Hz, 3H) | 155.22, 139.30, 130.18, 129.21, 128.66, 128.48, 127.53, 125.11, 120.70, 117.64, 50.97, 34.33, 22.32, 13.49 |
| 3c | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=10.0, 4.8 Hz, 3H), 7.26—7.11(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.90(m, 1H), 6.88—6.82(m, 1H), 5.35(s, 1H), 2.49—2.42(m, 2H), 1.63—1.51(m, 2H), 1.38—1.33(m, 2H), 0.85(t, J=7.3 Hz, 3H) | 155.23, 139.29, 130.19, 129.21, 128.67, 128.51, 128.49, 128.46, 127.54, 125.11, 120.71, 117.64, 51.04, 32.02, 21.01, 21.96, 13.64 |
| 3d | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=9.7, 5.1 Hz, 3H), 7.28—7.13(m, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.96—6.88(m, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.45(tq, J=10.7, 5.3 Hz, 2H), 1.57(dd, J=14.7, 7.3 Hz, 2H), 1.31—1.26(m, 4H), 0.85(t, J=7.1 Hz, 3H) | 155.23, 139.31, 130.19, 129.21, 128.67, 128.51, 128.48, 128.46, 127.53, 125.12, 120.71, 117.63, 51.05, 32.33, 30.99, 28.61, 22.23, 13.94 |
| 3e | 7.40(d, J=7.5 Hz, 2H), 7.31(dd, J=9.9, 4.9 Hz, 3H), 7.27—7.17(m, 2H), 7.03(dd, J=7.6, 1.2 Hz, 1H), 6.92(dd, J=8.0, 0.6 Hz, 1H), 6.86—6.82(m, 1H), 5.34(s, 1H), 2.45(tq, J=10.8, 5.3 Hz, 2H), 1.60—1.56(m, 2H), 1.32—1.23(m, 8H), 0.86(t, J=6.9 Hz, 3H) | 155.25, 139.29, 130.18, 129.21, 128.66, 128.54, 128.49, 127.53, 125.09, 120.70, 117.65, 51.08, 32.34, 31.67, 28.92, 28.80, 28.77, 22.61, 14.09 |
| 3f | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=13.8, 6.6 Hz, 3H), 7.21(ddd, J=11.3, 8.7, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.92(dd, J= 8.0, 0.6 Hz, 1H), 6.87—6.80(m, 1H), 5.31(s, 1H), 2.35(qd, J=12.5, 6.8 Hz, 2H), 1.83(dt, J=13.4, 6.7 Hz, 1H), 0.95(dd, J=8.0, 6.8 Hz, 6H) | 155.26, 139.36, 130.24, 129.22, 128.66, 128.51, 128.48, 128.45, 127.54, 125.12, 120.69, 117.65, 51.54, 41.27, 28.24, 22.22, 21.98 |
| 3g | 7.40(d, J=7.4 Hz, 2H), 7.36—7.26(m, 3H), 7.21(ddd, J=17.1, 11.2, 4.3 Hz, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.92(d, J=7.7 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.48—2.43(m, 2H), 1.61(dt, J=13.3, 6.6 Hz, 1H), 1.50—1.45(m, 2H), 0.84—0.81(m, 6H) | 155.22, 139.26, 130.19, 129.23, 128.66, 128.51, 128.49, 128.46, 127.54, 125.06, 120.71, 117.64, 51.03, 37.92, 30.36, 27.40, 22.32, 22.15 |
| 3h | 7.49—7.34(m, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.14(m, 2H), 7.04(dd, J=7.6, 1.2 Hz, 1H), 6.92(d, J=8.0 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.41(s, 1H), 2.77(dt, J=13.4, 6.7 Hz, 1H), 1.29—1.24(m, 6H) | 155.30, 139.28, 130.09, 129.19, 128.67, 128.48, 127.50, 125.11, 120.73, 117.73, 50.13, 35.76, 23.07, 22.85 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3i | 7.40(d, J=7.3 Hz, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.13(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.88(m, 1H), 6.85(dd, J=10.8, 4.1 Hz, 1H), 5.36(s, 1H), 2.91(p, J=7.0 Hz, 1H), 1.91(dt, J=12.3, 6.3 Hz, 2H), 1.72—1.70(m, 2H), 1.64—1.50(m, 4H) | 155.33, 139.37, 130.05, 129.15, 128.64, 128.46, 128.44, 127.47, 125.36, 120.70, 117.71, 51.40, 44.23, 33.65, 33.20, 24.88, 24.74 |
| 3j | 7.36(d, J=7.3 Hz, 2H), 7.30(t, J=7.3 Hz, 4H), 7.27—7.22(m, 2H), 7.18(dd, J=12.7, 4.5 Hz, 3H), 6.95(dd, J=7.7, 1.3 Hz, 1H), 6.90(d, J= 8.4 Hz, 2H), 6.86—6.79(m, 1H), 5.11(s, 1H), 3.59(d, J=3.2 Hz, 2H) | 155.03, 138.75, 137.28, 130.31 , 129.30, 129.01, 128.99, 128.76, 128.73, 127.65, 127.47, 124.86, 120.80, 117.60, 49.68, 36.54 |
| 3k | 7.34(dt, J=14.9, 7.8 Hz, 6H), 7.27—7.19(m, 2H), 7.15(d, J=8.2 Hz, 2H), 6.99(s, 1H), 6.96—6.90(m, 2H), 6.88—6.79(m, 1H), 5.14(s, 1H), 3.59(d, J=2.5 Hz, 2H), 1.32(s, 9H) | 155.14, 150.39, 138.73, 134.04, 130.35, 129.27, 128.73, 128.67, 128.64, 127.59, 125.59, 124.78, 120.70, 117.64, 49.97, 36.13, 34.57, 31.37 |
| 3l | 7.36—7.18(m, 8H), 7.11(d, J=8.4 Hz, 2H), 6.99(dd, J=7.6, 1.3 Hz, 1H), 6.93—6.81(m, 2H), 6.78(s, 1H), 5.12(s, 1H), 3.56(s, 2H) | 154.87, 138.61, 135.81, 133.18, 130.32, 130.29, 129.35,128.81, 128.75, 128.64, 127.70, 124.63, 120.88, 117.52, 49.65, 35.89 |
| 3m | 7.40—7.29(m, 4H), 7.26—7.17(m, 2H), 7.13—7.08(m, 4H), 6.96(s, 1H), 6.93—6.90(m, 2H), 6.83(t, J=7.4 Hz, 1H), 5.09(s, 1H), 3.56(d, J=5.1 Hz, 2H), 2.33(s, 3H) | 155.08, 138.74, 137.14, 134.11, 130.31, 129.41, 129.26 , 128.90, 128.79, 128.71, 127.61, 124.87, 120.74, 117.62, 49.64, 36.21, 21.20 |
| 3n | 7.61(s, 1H), 7.42(d, J=7.4 Hz, 2H), 7.34(t, J=7.4 Hz, 2H), 7.30—7.21(m, 2H), 6.74(dd, J=7.2, 5.3 Hz, 2H), 5.35(s, 1H), 2.46(q, J=7.4 Hz, 2H), 1.44(s, 9H), 1.25(t, J=7.3 Hz, 3H) | 154.38, 138.88, 138.29, 128.82, 128.63, 128.04, 127.56, 126.66, 125.19, 119.80, 77.36, 77.05, 76.73, 51.54, 34.93, 29.76, 26.27, 14.24 |
| 3o | 7.47(d, J=7.4 Hz, 2H), 7.28(t, J=7.5 Hz, 2H), 7.22—7.08(m, 2H), 6.81(t, J=8.0 Hz, 1H), 6.71(dd, J=8.0, 1.1 Hz, 1H), 5.98(s, 1H), 5.66(s, 1H), 4.10—4.02(m, 2H), 2.44(q, J=7.4 Hz, 2H), 1.41(t, J=7.0 Hz, 3H), 1.23(t, J=7.4 Hz, 3H) | 145.71, 143.18, 141.48, 128.38, 128.35, 127.33, 126.88, 121.02, 119.63, 110.09, 64.57, 46.17, 26.31, 14.92, 14.31 |
| 3p | 7.40(d, J=7.5 Hz, 2H), 7.31(t, J=7.4 Hz, 2H), 7.26—7.22(m, 1H), 7.12(s, 1H), 7.00(dd, J=8.2, 1.7 Hz, 1H), 6.83(dd, J=8.1, 4.9 Hz, 2H), 5.34(s, 1H), 2.48(dd, J=7.4, 4.0 Hz, 2H), 2.21(s, 3H), 1.25(t, J=7.4 Hz, 3H) | 152.92, 139.33, 130.56, 129.85, 129.72, 128.65, 128.45, 127.48, 124.56, 117.49, 50.76, 26.36, 20.57, 14.16 |
| 3q | 7.41(d, J=7.4 Hz, 2H), 7.32(dd, J=10.1, 4.7 Hz, 2H), 7.25(t, J=7.2 Hz, 1H), 6.86(d, J=8.8 Hz, 1H), 6.76(dd, J=8.8, 3.1 Hz, 2H), 6.63(d, J=3.0 Hz, 1H), 5.34(s, 1H), 3.70(s, 3H), 2.48(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 153.54, 148.85, 139.05, 128.66, 128.64, 128.49, 128.44, 127.56, 126.25, 118.26, 115.74, 113.95, 55.66, 50.59, 26.35, 14.18 |
| 3r | 7.54—7.51(m, 1H), 7.29(s, 1H), 7.23—7.11(m, 4H), 6.94(ddd, J=11.9, 7.9, 1.0 Hz, 2H), 6.83—6.80(m, 1H), 5.52(s, 1H), 2.51(q, J=7.4 Hz, 2H), 2.39(s, 3H), 1.27(t, J=7.4 Hz, 3H) | 155.18, 137.18, 136.28, 130.73, 129.94, 128.98, 128.62, 127.48, 126.47, 125.15, 120.79, 117.44, 46.90, 26.47, 19.31, 14.16 |
| 3s | 7.34(d, J=8.5 Hz, 2H), 7.22(ddd, J=11.3, 8.7 Hz, 4.7 Hz, 2H), 7.08—7.03(m, 2H), 6.93—6.85(m, 2H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 154.95, 137.91, 133.29, 129.97, 129.84, 129.35, 128.78, 124.75, 120.86, 117.63, 49.68, 26.37, 14.12 |
| 3t | 7.30—7.26(m, 1H), 7.21(ddd, J=12.9, 9.8, 4.7 Hz, 2H), 7.14(dd, J=10.1, 2.0 Hz, 1H), 7.06(dd, J=7.6, 1.3 Hz, 1H), 7.01(s, 1H), 6.97—6.86(m, 3H), 5.38(s, 1H), 2.49(qd, J=7.4, 1.6 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 164.17, 161.72, 154.88, 142.07(d, J=6.9 Hz), 130.07(d, J=8.3 Hz), 129.99, 129.37, 124.76, 124.12(d, J=2.8 Hz), 120.89, 117.58, 115.63, 115.41, 114.56, 114.35, 49.70, 26.36, 14.12 |
| 3u | 7.39—7.35(m, 2H), 7.22(td, J=8.1, 1.6 Hz, 1H), 7.17(s, 1H), 7.05—6.98(m, 3H), 6.93(dd, J =8.0, 0.8 Hz, 1H), 6.87(td, J=7.5, 1.0 Hz, 1H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.7 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 162.04(d, J=245 Hz), 155.05, 134.99(d, J=3 Hz), 130.12, 130.03(d, J=2.6 Hz), 129.32, 124.87, 120.80, 117.69, 115.59, 115.38, 49.78, 26.35, 14.10 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3v | 7.40—7.25(m, 6H), 7.16(dd, J=8.6, 2.6 Hz, 1H), 7.03(d, J=2.5 Hz, 1H), 6.86(d, J=8.6 Hz, 1H), 5.31(s, 1H), 2.49(dd, J=13.7, 7.0 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 153.82, 138.45, 129.70, 129.03, 128.80, 128.76, 128.41, 128.36, 128.32, 127.82, 126.81, 125.44, 119.00, 50.32, 29.71, 26.41, 14.10 |
| 3w | 7.64(s, 1H), 7.18—7.14(m, 1H), 6.96(dd, J=7.5, 1.3 Hz, 1H), 6.86(d, J=7.7, 1H), 6.80(dd, J=11.5, 4.1 Hz, 1H), 3.78(d, J=9.1 Hz, 1H), 2.29(qd, J=7.4, 1.5 Hz, 2H), 2.14(d, J=12.8 Hz, 1H), 1.87—1.85(m, 1H), 1.74(dd, J=10.4, 2.7 Hz, 1H), 1.63—1.60(m, 2H), 1.41(d, J=13.0 Hz, 1H), 1.23(ddd, J=15.7, 8.6, 5.2 Hz, 1H), 1.15—1.03(m, 6H), 0.92—0.90(m, 1H) | 155.51, 131.14, 128.68, 124.72, 119.88, 117.59, 55.16, 41.45, 32.07, 32.02, 26.28, 26.16, 14.24 |
| 3x | 7.20(t, J=7.5 Hz, 1H), 7.08(d, J=7.2 Hz, 1H), 6.97—6.79(m, 2H), 6.75(s, 1H), 3.82(s, 2H), 2.42(q, J=7.3 Hz, 2H), 1.26—1.21(m, 3H) | 155.51, 130.45, 129.11, 122.44, 120.52, 117.20, 32.38 , 24.76, 14.23 |
| 4a | 7.40(d, J=7.4 Hz, 2H), 7.25(td, J=17.2, 7.3 Hz, 5H), 6.74(d, J=8.5 Hz, 2H), 5.79(s, 1H), 5.12(s, 1H), 2.37(q, J=7.4 Hz, 2H), 1.19(t, J=7.4 Hz, 3H) | 154.72, 141.81, 133.61, 129.56, 128.56, 128.26, 127.09, 115.49, 53.17, 26.27, 14.25 |
| [1] | Kondo T., Mitsudo T. A., Chem.Rev., 2000, 100(8), 3205—3220 |
| [2] | Feng M., Tang B., Liang S. H., Jiang X., Curr. Top. Med.Chem.,2016, 16(17), 1200—1216 |
| [3] | Ilardi E. A., Vitaku E., Najardarson J. T., J. Med.Chem., 2014, 57(7), 2832—2842 |
| [4] | Smith B. R., Eastman C. M., Nijardarson., J. Med.Chem., 2014, 57(23), 9764—9773 |
| [5] | Wei Y. Z., Tan X. Z., Chem. Res. Chinese Universities,2017, 33(5), 731—735 |
| [6] | Zhong S. M., Zheng M., Pu S. X., Xing Y. B., Li K. Z., Wang H., Chem. Res. Chinese Universities,2017, 33(6), 979—985 |
| [7] | Cinar M. E., Ozturk T., Chem.Rev., 2015, 115(9), 3036—3140 |
| [8] | Takimiya K., Osaka I., Mori T., Nakano M., Acc. Chem.Res., 2014, 47(5), 1493—1502 |
| [9] | Scott K. A., Njardarson J. T., Top. Curr.Chem., 2018, 376, 5—38 |
| [10] | Hammond M. L., Zambias R. A., Chang M. N., Jensen N. P., McDonald J., Thompson K., Boulton D. A., Kopka I. K., Hand K. M., Opas E. E., Luell S., Bach T., Davies P., MacIntyre D. E., Bonney R. J., Humes J. L., J. Med.Chem.,1990, 33(3), 908—918 |
| [11] | Katritzky A. R., Zhang Z., Lan X., Lang H., J. Org.Chem., 1994, 59(7), 1900—1903 |
| [12] | Modica E., Zanaletti R., Freccero M., Mella M., J. Org.Chem.,2001, 66(1), 41—52 |
| [13] | Shaikh A. K., Cobb A. J. A., Varvounis G., Org.Lett., 2012, 14(2), 584—587 |
| [14] | Lau C. K., Williams H. W. R., Tardiff S., Dufresne C., Scheigetz J., Belanger P., Can. J.Chem., 1989, 67, 1384—1387 |
| [15] | Guo W., Wu B., Zhou X., Chen P., Wang X., Zhou Y. G., Liu Y., Li C., Angew. Chem. Int.Ed., 2015, 54(15), 4522—4526 |
| [16] | Guo W., Wu B., Zhou X., Chen P., Wang X., Zhou Y. G., Liu Y., Li C.,Angew. Chem. Int.Ed., 2015, 54(15), 4605—4609 |
| [17] | Lai Z., Sun J., Synlett.,2016, 27(4), 555—558 |
| [18] | Pathak T. P., Sigman M. S., J. Org.Chem.,2011, 76(22), 9210—9215 |
| [19] | Will N. J., Bray C. D., Chem. Eur.J.,2012, 18(30), 9160—9173 |
| [20] | Caruana L., Fochi M., Bernardi L., Molecules,2015, 20(7), 11733—11764 |
| [21] | Wang Z., Sun J., Synthesis,2015, 47(23), 3629—3644 |
| [22] | Water R. W. V. D., Pettus T. R. R., Tetrahedron,2002, 58(27), 5367—5405 |
| [23] | Kulikov A., Arumugam S., Popik V. V., J. Org.Chem.,2008, 73(19), 7611—7615 |
| [24] | Mattson A. E., Scheidt K. A., J. Am. Chem.Soc.,2007, 129(15), 4508—4509 |
| [25] | Luan Y., Schaus S. E., J. Am. Chem.Soc., 2012, 134(49), 19965—19968 |
| [26] | Chen M. W., Gao L. L., Ye Z. S., Jiang G. F., Zhou Y. G., Chem.Commun.,2013, 49, 1660—1662 |
| [27] | Bai W. J., David J. G., Feng Z. G., Weaver M. G., Wu K. L., Pettus T. R. R., Acc. Chem.Res., 2014, 47(12), 3655—3664 |
| [28] | Zhao W., Wang Z., Chu B., Sun J., Angew. Chem. Int.Ed., 2015, 54(6), 1910—1913 |
| [29] | Huang Y., Hayashi T., J. Am. Chem.Soc.,2015, 137(24), 7556—7559 |
| [30] | Wan Z., Ai F., Wang Z., Zhao W., Zhu G., Lin Z., Sun J., J. Am. Chem.Soc., 2015, 137(1), 383—389 |
| [31] | Wu B., Yu Z., Gao X., Lan Y., Zhou Y. G., Angew. Chem. Int.Ed.,2017, 56(14), 4006—4010 |
| [32] | Chen P., Wang K., Guo W., Liu X., Liu Y., Li C., Angew. Chem. Int.Ed.,2017, 56(13), 3689—3693 |
| [33] | Nising C. F., Brase S., Chem. Soc.Rev., 2008, 37, 1218—1228 |
| [34] | Nising C. F., Brase S., Chem. Soc.Rev., 2012, 41, 988—999 |
| [35] | Heravi M. M., Hajiabbasi P., Mol.Diversity,2014, 18(2), 411—439 |
| [36] | Thirupathi N., Tung C. H., Xu Z. H.,Adv. Synth.Catal., 2018, 360(18), 3585—3589 |
| [37] | Corma A., Gonzalez-Arellano C., Iglesias M., Sanchez F., App. Catal. A:Gen.,2010, 375(1), 49—54 |
| [38] | Lee W. Z., Tseng H. S., Wang T. L., Tsai H. L., Kuo T. S.,Organometallics,2010, 29(13), 2874—2881 |
| [39] | Badoiu A., Bernardinelli G., Besnard C., Kundig E. P., Org. Biomol.Chem., 2010, 8, 193—200 |
| [40] | O’Byrne A., Murray C., Keegan D., Palacio C., Evans P., Morgan B. S., Org. Biomol.Chem., 2010, 8, 539—545 |
| [41] | Firouzabadi H., Iranpoor N., Abbasi M., Adv. Synth.Catal., 2009, 351, 755—766 |
| [42] | Rajabi F., Razavi S., Luque R., Green Chem.,2010, 12, 786—789 |
| [43] | Inoue T., Inoue S., Sato K., Bull. Chem. Soc.Jpn.,1990, 63(4), 1062—1068 |
| [44] | Lanzi M., Merad J., Boyarskaya D V., Maestri G., Allain C., Masson Géraldine., Org.Lett., 2018, 20(17), 5247—5250 |
| [45] | Basha R. S., Chen C. W., Reddy D. M., Lee C. F., Chem-Asian J.,2018,13(17), 2475—2483 |
| [1] | 李艾橘, 王玉玺, 卢少勇, 刘堃. 硫醇末端基聚合物对金纳米粒子配体置换[J]. 高等学校化学学报, 2018, 39(3): 552. |
| [2] | 于海丰, 廖沛球. 肟的无气味硫缩醛/酮化反应[J]. 高等学校化学学报, 2012, 33(09): 1969. |
| [3] | 曾智 关绍巍 祝世洋 代中明 姜振华. 新型芳基硫醇的合成及在聚芳醚砜紫外交联中的应用[J]. 高等学校化学学报, 2011, 32(2): 407. |
| [4] | 谢斌, 张秀兰, 邹立科, 王军, 赖川, 吴宇, 冯建申. 配合物{[Cu(hmtade)][Ni(dmit)2]}2·4DMSO的合成、表征及晶体结构[J]. 高等学校化学学报, 2009, 30(12): 2337. |
| [5] | 刘艳芝, 施小宁, 李志锋, 唐慧安, 袁焜, 张俊彦. CH3SH…HOO开壳型氢键复合物的结构及其电子密度拓扑性质[J]. 高等学校化学学报, 2009, 30(10): 2049. |
| [6] | 祝纶宇,许凯,王洪,刘鹏,陈德宏,艾好,陈鸣才 . 稀土(镧)硫醇盐的合成与表征[J]. 高等学校化学学报, 2008, 29(9): 1786. |
| [7] | 张晓鹏,陆世维 . 硒作用下苯胺和硫醇羰基化合成硫代氨基甲酸酯[J]. 高等学校化学学报, 2008, 29(6): 1137. |
| [8] | 于海丰,王艳茹,欧阳艳,王岩,刘群 . 4,4-二乙硫/苄硫基-3-烯-2-丁酮的合成及其作为无气味硫醇替代试剂的缩硫醛/酮化反应[J]. 高等学校化学学报, 2006, 27(12): 2300. |
| [9] | 欧阳艳, 于海丰, 董德文, 刘军, 王芒, 刘群. 2-(1,3-亚丙二硫)亚甲基-3-羰基丁酸作为硫醇替代试剂在缩硫醛/酮化反应中的应用[J]. 高等学校化学学报, 2005, 26(12): 2237. |
| [10] | 欧阳艳 于海丰 董德文 刘军 王芒 刘群. 2-(1,3-亚丙二硫)亚甲基-3-羰基丁酸作为硫醇替代试剂在缩硫醛/酮化反应中的应用[J]. 高等学校化学学报, 2005, 26(12): 2237. |
| [11] | 龚少云, 沈艳飞, 周伟红, 李景虹. 硫醇自组装膜对金电极上吡咯电化学聚合的影响[J]. 高等学校化学学报, 2004, 25(9): 1719. |
| [12] | 刘福胜, 于世涛, 葛晓萍, 杨锦宗. 2位取代嘌呤衍生物的合成研究[J]. 高等学校化学学报, 2003, 24(9): 1592. |
| [13] | 刘建兵, 赵国锋, 李焜昶, 金桂玉. 1-(2,4-二氯苯基)-1-氨基-4,4-二甲基-2-(1,2,4-三唑-1-基)-3-戊酮(醇)的合成及生物活性研究[J]. 高等学校化学学报, 2001, 22(S1): 92. |
| [14] | 陈文彬, 金桂玉. 新型含硫三唑类化合物的合成及生物活性研究[J]. 高等学校化学学报, 2001, 22(7): 1147. |
| [15] | 杨生荣, 任嗣利, 张俊彦, 张绪寿. 自组装单分子膜的结构及其自组装机理[J]. 高等学校化学学报, 2001, 22(3): 470. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||