

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (11): 1977.doi: 10.7503/cjcu20160434
秦耀果1, 张景朋1, 宋敦伦2, 段红霞1, 凌云1, 蒋标标1, 王迪1, 杨新玲1( )
)
收稿日期:2016-06-15
出版日期:2016-11-10
发布日期:2016-10-18
作者简介:联系人简介: 杨新玲, 女, 博士, 教授, 博士生导师, 主要从事新农药分子设计与创制方面的研究. E-mail:基金资助:
QIN Yaoguo1, ZHANG Jingpeng1, SONG Dunlun2, DUAN Hongxia1, LING Yun1, JIANG Biaobiao1, WANG Di1, YANG Xinling1,*( )
)
Received:2016-06-15
Online:2016-11-10
Published:2016-10-18
Contact:
YANG Xinling
E-mail:yangxl@cau.edu.cn
Supported by:摘要:
以蚜虫报警信息素[(E)-β-farnesene, EBF]为先导, 通过活性亚结构拼接和生物电子等排原理, 设计合成了一系列结构新颖的异烟酸类EBF类似物. 以香叶醇为原料, 经4步反应制得20个目标化合物(19个未见文献报道), 其结构经1H NMR, 13C NMR, IR及HRMS确证. 初步生物活性测定结果表明, 所有化合物对桃蚜有驱避活性和杀死活性, 其中化合物7d, 8f和8n表现出较好的桃蚜驱避活性, 对桃蚜的驱避率分别为62.6%, 62.0%和61.0%; 化合物8a, 8b和8d对桃蚜的致死率分别为73.6%, 81.1%和70.2%. 初步构效关系分析发现, 酯基的引入对桃蚜的驱避活性有利; 酰胺基的引入对杀蚜活性有利; N烷基取代的链长及支链数量影响驱避活性.
中图分类号:
TrendMD:
秦耀果, 张景朋, 宋敦伦, 段红霞, 凌云, 蒋标标, 王迪, 杨新玲. 新型异烟酸类蚜虫报警信息素类似物的设计、 合成及生物活性. 高等学校化学学报, 2016, 37(11): 1977.
QIN Yaoguo, ZHANG Jingpeng, SONG Dunlun, DUAN Hongxia, LING Yun, JIANG Biaobiao, WANG Di, YANG Xinling. Design, Synthesis and Biological Activity of Novel Aphid Alarm Pheromone Analogues Containing Isonicotinic Acid†. Chem. J. Chinese Universities, 2016, 37(11): 1977.
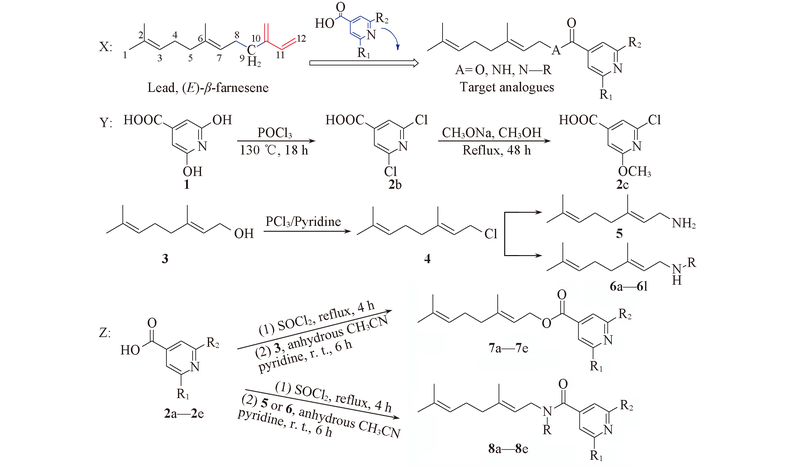
Scheme 1 Design strategy(X), general synthetic route of intermediates(Y) and target compounds(Z)2a, 7a: R1=H, R2=H; 2b, 7b: R1=Cl, R2=Cl; 2c, 7c: R1=Cl, R2=OCH3; 2d, 7d: R1=Cl, R2=H; 2e, 7e: R1=OCH3, R2=H; 6a: R=CH3; 6b: R=CH2CH3; 6c: R=(CH2)2CH3; 6d: R =(CH2)3CH3; 6e: R=(CH2)4CH3; 6f: R=(CH2)5CH3; 6g: R=CH(CH3)2; 6h: R=CH2CH(CH3)2; 6i: R=C(CH3)3; 6j: R=A; 6k: R=B; 6l: R=C; 8a: R1=H, R2=H, R=H; 8b: R1=Cl, R2=Cl, R=H; 8c: R1=Cl, R2=OCH3, R=H; 8d: R1=Cl, R2=Cl, R=CH3; 8e: R1=Cl, R2=Cl, R=CH2CH3; 8f: R1=Cl, R2=Cl, R=(CH2)2CH3; 8g: R1=Cl, R2=Cl, R=(CH2)3CH3; 8h: R1=Cl, R2=Cl, R=(CH2)4CH3; 8i: R1=Cl, R2=Cl, R=(CH2)5CH3; 8j: R1=Cl, R2=Cl, R=CH(CH3)2; 8k: R1=Cl, R2=Cl, R=CH2CH(CH3)2; 8l: R1=Cl, R2=Cl, R=C(CH3)3; 8m: R1=Cl, R2=Cl, R=A; 8n: R1=Cl, R2=Cl, R=B; 8o: R1=Cl, R2=Cl, R=C. A= ; B=; C=.
| Compd. | Chemical formula | MS(calcd.), m/z | Compd. | Chemical formula | MS(calcd.), m/z |
|---|---|---|---|---|---|
| 4 | C11H20O | 169.1(168.1) | 6f | C16H31N | 238.2(237.2) |
| 5 | C10H19N | 154.2(153.2) | 6g | C13H25N | 196.2(195.2) |
| 6a | C11H21N | 168.2(167.2) | 6h | C14H27N | 210.2(209.2) |
| 6b | C12H23N | 182.2(181.2) | 6i | C14H27N | 210.2(209.2) |
| 6c | C13H25N | 196.2(195.2) | 6j | C16H29N | 236.2(235.2) |
| 6d | C14H27N | 210.2(209.2) | 6k | C16H23N | 230.2(229.2) |
| 6e | C15H29N | 224.2(223.2) | 6l | C17H25N | 244.2(243.2) |
Table 1 MS data of intermediates 4—6*
| Compd. | Chemical formula | MS(calcd.), m/z | Compd. | Chemical formula | MS(calcd.), m/z |
|---|---|---|---|---|---|
| 4 | C11H20O | 169.1(168.1) | 6f | C16H31N | 238.2(237.2) |
| 5 | C10H19N | 154.2(153.2) | 6g | C13H25N | 196.2(195.2) |
| 6a | C11H21N | 168.2(167.2) | 6h | C14H27N | 210.2(209.2) |
| 6b | C12H23N | 182.2(181.2) | 6i | C14H27N | 210.2(209.2) |
| 6c | C13H25N | 196.2(195.2) | 6j | C16H29N | 236.2(235.2) |
| 6d | C14H27N | 210.2(209.2) | 6k | C16H23N | 230.2(229.2) |
| 6e | C15H29N | 224.2(223.2) | 6l | C17H25N | 244.2(243.2) |
| Compd. | Appearance | Yield(%) | Chemical formula | ESI-HRMSb(calcd.), m/z | IR(KBr), | |
|---|---|---|---|---|---|---|
| 7a | Yellow liquid | 85.9 | C16H21NO2 | 260.1646(260.1645) | 3031, 2967, 2918, 2857, 1729, 1408, 1278, 1119, 930, 850 | |
| 7b | Yellow liquid | 85.3 | C16H19Cl2NO2 | 328.0867(328.0866) | 3088, 2967, 2926, 2856, 1732, 1582, 1548, 1442, 1358, 1277, 1155, 933, 816 | |
| 7c | Yellow liquid | 70.3 | C17H22ClNO3 | 324.1364(324.1361) | 3091, 2951, 2925, 2856, 1732, 1604, 1555, 1463, 1384, 1357, 1317, 1265, 1153, 1042, 938, 887 | |
| 7d | Yellow liquid | 74.5 | C16H20ClNO2 | 294.1259(294.1255) | 3064, 2968, 2918, 2857, 1731, 1591, 1552, 1462, 1368, 1291, 1259, 1145, 1107, 931, 860 | |
| 7e | Yellow liquid | 63.2 | C17H23NO3 | 290.1750(290.1751) | 2968, 2925, 2857, 1731, 1674, 1611, 1564, 1553, 1449, 1386, 1292, 1259, 1215, 1039, 935, 840 | |
| 8a | Yellow liquid | 63.8 | C16H22N2O | 259.1806(259.1805) | 3304, 3045, 2965, 2924, 2855, 1645, 1600, 1542, 1490, 1449, 1297, 1066, 846 | |
| 8b | White solid | 85.5 | C16H20Cl2N2O | 327.1026(327.1025) | 3273, 3129, 3083, 2964, 2924, 2854, 1644, 1447, 1358, 1294, 1158, 820 | |
| 8c | White solid | 69.5 | C17H23ClN2O2 | 323.1519(323.1521) | 3273, 2924, 2856, 1645, 1603, 1538, 1463, 1379, 1162, 1047, 873 | |
| 8d | Yellow liquid | 68.5 | C17H22Cl2N2O | 363.1000(363.1001) | 3270, 3068, 2971, 2930, 2856, 1644, 1583, 1533, 1455, 1381, 1309, 1286, 1264, 1164, 873, 822 | |
| 8e | Yellow liquid | 83.2 | C18H24Cl2N2O | 355.1339(355.1338) | 3270, 3068, 2971, 2930, 2856, 1644, 1583, 1533, 1455, 1361, 1164, 873, 822 | |
| 8f | Yellow liquid | 91.9 | C19H26Cl2N2O | 369.1496(369.1495) | 3269, 3068, 2966, 2930, 2876, 1643, 1583, 1534, 1437, 1406, 1361, 1261, 1164, 872, 822 | |
| 8g | Yellow liquid | 68.6 | C20H28Cl2N2O | 405.1472(405.1471) | 3068, 2963, 2928, 2872, 1643, 1583, 1534, 1436, 1361, 1290, 1266, 1164, 872, 822 | |
| 8h | Yellow liquid | 59.3 | C21H30Cl2N2O | 419.1628(419.1627) | 3068, 2960, 2930, 2859, 1642, 1583, 1534, 1438, 1360, 1263, 1163, 872, 822 | |
| 8i | Yellow liquid | 91.8 | C22H32Cl2N2O | 411.1967(411.1964) | 3068, 2958, 2929, 2858, 1642, 1583, 1534, 1438, 1360, 1264, 1163, 872, 822 | |
| 8j | Yellow liquid | 83.7 | C19H26Cl2N2O | 369.1496(369.1495) | 3066, 2973, 2929, 1638, 1582, 1534, 1449, 1406, 1360, 1164, 872, 823 | |
| 8k | Yellow liquid | 90.7 | C20H28Cl2N2O | 383.1655(383.1651) | 3068, 2962, 2930, 2873, 1642, 1583, 1534, 1438, 1383, 1360, 1164, 872, 822 | |
| 8l | Yellow liquid | 80.0 | C20H28Cl2N2O | 383.1649(383.1651) | 3068, 2967, 2925, 1645, 1580, 1534, 1421, 1361, 1261,1164, 871, 823 | |
| 8m | Yellow liquid | 60.1 | C22H30Cl2N2O | 409.1808(409.1808) | 3068, 2931, 2856, 1714, 1634, 1582, 1533, 1453, 1405, 1361, 1253, 1164, 1102, 871, 822 | |
| 8n | Yellow liquid | 67.3 | C22H24Cl2N2O | 403.1336(403.1338) | 3066, 2967, 2924, 2855, 1654, 1594, 1582, 1536, 1494, 1420, 1384, 1359, 1293, 1164, 874, 819 | |
| 8o | Yellow liquid | 91.8 | C23H26Cl2N2O | 417.1493(417.1495) | 3066, 3030, 2967, 2924, 2855, 1643, 1583, 1535, 1496, 1451, 1360, 1263, 1164, 872, 822 | |
Table 2 Appearance, yields, HRMS and IR data of target compounds 7 and 8a
| Compd. | Appearance | Yield(%) | Chemical formula | ESI-HRMSb(calcd.), m/z | IR(KBr), | |
|---|---|---|---|---|---|---|
| 7a | Yellow liquid | 85.9 | C16H21NO2 | 260.1646(260.1645) | 3031, 2967, 2918, 2857, 1729, 1408, 1278, 1119, 930, 850 | |
| 7b | Yellow liquid | 85.3 | C16H19Cl2NO2 | 328.0867(328.0866) | 3088, 2967, 2926, 2856, 1732, 1582, 1548, 1442, 1358, 1277, 1155, 933, 816 | |
| 7c | Yellow liquid | 70.3 | C17H22ClNO3 | 324.1364(324.1361) | 3091, 2951, 2925, 2856, 1732, 1604, 1555, 1463, 1384, 1357, 1317, 1265, 1153, 1042, 938, 887 | |
| 7d | Yellow liquid | 74.5 | C16H20ClNO2 | 294.1259(294.1255) | 3064, 2968, 2918, 2857, 1731, 1591, 1552, 1462, 1368, 1291, 1259, 1145, 1107, 931, 860 | |
| 7e | Yellow liquid | 63.2 | C17H23NO3 | 290.1750(290.1751) | 2968, 2925, 2857, 1731, 1674, 1611, 1564, 1553, 1449, 1386, 1292, 1259, 1215, 1039, 935, 840 | |
| 8a | Yellow liquid | 63.8 | C16H22N2O | 259.1806(259.1805) | 3304, 3045, 2965, 2924, 2855, 1645, 1600, 1542, 1490, 1449, 1297, 1066, 846 | |
| 8b | White solid | 85.5 | C16H20Cl2N2O | 327.1026(327.1025) | 3273, 3129, 3083, 2964, 2924, 2854, 1644, 1447, 1358, 1294, 1158, 820 | |
| 8c | White solid | 69.5 | C17H23ClN2O2 | 323.1519(323.1521) | 3273, 2924, 2856, 1645, 1603, 1538, 1463, 1379, 1162, 1047, 873 | |
| 8d | Yellow liquid | 68.5 | C17H22Cl2N2O | 363.1000(363.1001) | 3270, 3068, 2971, 2930, 2856, 1644, 1583, 1533, 1455, 1381, 1309, 1286, 1264, 1164, 873, 822 | |
| 8e | Yellow liquid | 83.2 | C18H24Cl2N2O | 355.1339(355.1338) | 3270, 3068, 2971, 2930, 2856, 1644, 1583, 1533, 1455, 1361, 1164, 873, 822 | |
| 8f | Yellow liquid | 91.9 | C19H26Cl2N2O | 369.1496(369.1495) | 3269, 3068, 2966, 2930, 2876, 1643, 1583, 1534, 1437, 1406, 1361, 1261, 1164, 872, 822 | |
| 8g | Yellow liquid | 68.6 | C20H28Cl2N2O | 405.1472(405.1471) | 3068, 2963, 2928, 2872, 1643, 1583, 1534, 1436, 1361, 1290, 1266, 1164, 872, 822 | |
| 8h | Yellow liquid | 59.3 | C21H30Cl2N2O | 419.1628(419.1627) | 3068, 2960, 2930, 2859, 1642, 1583, 1534, 1438, 1360, 1263, 1163, 872, 822 | |
| 8i | Yellow liquid | 91.8 | C22H32Cl2N2O | 411.1967(411.1964) | 3068, 2958, 2929, 2858, 1642, 1583, 1534, 1438, 1360, 1264, 1163, 872, 822 | |
| 8j | Yellow liquid | 83.7 | C19H26Cl2N2O | 369.1496(369.1495) | 3066, 2973, 2929, 1638, 1582, 1534, 1449, 1406, 1360, 1164, 872, 823 | |
| 8k | Yellow liquid | 90.7 | C20H28Cl2N2O | 383.1655(383.1651) | 3068, 2962, 2930, 2873, 1642, 1583, 1534, 1438, 1383, 1360, 1164, 872, 822 | |
| 8l | Yellow liquid | 80.0 | C20H28Cl2N2O | 383.1649(383.1651) | 3068, 2967, 2925, 1645, 1580, 1534, 1421, 1361, 1261,1164, 871, 823 | |
| 8m | Yellow liquid | 60.1 | C22H30Cl2N2O | 409.1808(409.1808) | 3068, 2931, 2856, 1714, 1634, 1582, 1533, 1453, 1405, 1361, 1253, 1164, 1102, 871, 822 | |
| 8n | Yellow liquid | 67.3 | C22H24Cl2N2O | 403.1336(403.1338) | 3066, 2967, 2924, 2855, 1654, 1594, 1582, 1536, 1494, 1420, 1384, 1359, 1293, 1164, 874, 819 | |
| 8o | Yellow liquid | 91.8 | C23H26Cl2N2O | 417.1493(417.1495) | 3066, 3030, 2967, 2924, 2855, 1643, 1583, 1535, 1496, 1451, 1360, 1263, 1164, 872, 822 | |
| Compd. | 1H NMR(300 MHz, CDCl3), δ | 13C NMR(75 MHz, CDCl3), δ | ||
|---|---|---|---|---|
| 7a | 8.71—8.73(m, 2H, ArH), 7.80—7.82(m, 2H, ArH), 5.37—5.42(m, 1H, C═CH), 5.03—5.05(m, 1H, C═CH), 4.83(d, J=7.1 Hz, 2H, CH2), 2.00—2.12(m, 4H, CH2CH2), 1.74(s, 3H, CH3), 1.63(s, 3H, CH3), 1.56(s, 3H, CH3) | 164.62, 150.06, 142.86, 137.35, 131.42, 123.24, 122.48, 117.36, 62.18, 39.15, 25.84, 25.22, 17.25, 16.13 | ||
| 7b | 7.80—7.81(m, 2H, ArH), 5.41—5.46(m, 1H, C═CH), 5.06—5.09(m, 1H, C═CH), 4.88(d, J=7.3 Hz, 2H, CH2), 2.07—2.16(m, 4H, CH2CH2), 1.68—1.77 [m, 6H, C(CH3)2], 1.61(s, 3H, CH3) | 162.24, 151.00, 143.75, 142.53, 131.51, 123.17, 122.24, 116.80, 62.94, 39.13, 25.83, 25.25, 17.29, 16.19 | ||
| 7c | 7.44(d, J=1.1 Hz, 1H, ArH), 7.23(d, J=1.1 Hz, 1H, ArH), 5.43—5.48(m, 1H, C═CH), 5.07—5.12(m, 1H, C═CH), 4.86(d, J=7.2 Hz, 2H, CH2), 3.97(s, 3H, OCH3), 2.05—2.17(m, 4H, CH2CH2), 1.78(s, 3H, CH3), 1.69(s, 3H, CH3), 1.62(s, 3H, CH3) | 163.99, 163.47, 148.80, 143.14, 142.46, 131.53, 123.24, 117.19, 115.35, 109.30, 62.42, 54.07, 39.15, 25.87, 25.27, 17.30, 16.18 | ||
| 7d | 8.53(dd, J1=5.1 Hz, J2=0.7 Hz, 1H, ArH), 7.89(dd, J1=1.3 Hz, J2=0.8 Hz, 1H, ArH), 7.78(dd, J1=5.1 Hz, J2=1.4 Hz, 1H, ArH), 5.42—5.48(m, 1H, C═CH), 5.06—5.11(m, 1H, C═CH), 4.88(d, J=7.2 Hz, 2H, CH2), 2.07—2.16(m, 4H, CH2CH2), 1.78(s, 3H, CH3), 1.68(s, 3H, CH3), 1.61(s, 3H, CH3) | 163.23, 151.93, 149.98, 143.18, 140.23, 131.35, 123.54, 123.18, 121.19, 117.08, 62.47, 39.08, 25.79, 25.19, 17.22, 16.10 | ||
| 7e | 8.29(dd, J1=5.3 Hz, J2=0.6 Hz, 1H, ArH), 7.43(dd, J1=5.3 Hz, J2=1.4 Hz, 1H, ArH), 7.34(d, J=0.4 Hz, 1H, ArH), 5.44—5.49(m, 1H, C═CH), 5.08—5.13(m, 1H, C═CH), 4.87(d, J=7.1 Hz, 2H, CH2), 3.98(s, 3H, OCH3), 2.06—2.17(m, 4H, CH2CH2), 1.79(s, 3H, CH3), 1.70(s, 3H, CH3), 1.62(s, 3H, CH3) | 164.68, 164.47, 147.24, 142.79, 140.20, 131.53, 123.29, 117.45, 115.41, 110.87, 62.12, 53.42, 39.17, 25.91, 25.29, 17.32, 16.20 | ||
| 8a | 8.71—8.73(m, 2H, ArH), 7.60—7.62(m, 2H, ArH), 6.28(brs, 1H, NH), 5.26—5.31(m, 1H, C═CH), 5.06—5.10(m, 1H, C═CH), 4.04—4.13(m, 2H, CH2), 2.01—2.11(m, 4H, CH2CH2), 1.68—1.72 [m, 6H, C(CH3)2], 1.60(s, 3H, CH3) | 165.09, 149.63, 141.58, 139.27, 131.16, 123.31, 120.87, 119.19, 39.03, 37.76, 25.92, 25.18, 17.19, 15.82 | ||
| 8b | 7.57(s, 2H, ArH), 6.08(brs, 1H, NH), 5.24—5.29(m, 1H, C═CH), 5.06—5.08(m, 1H, C═CH), 4.05(t, J=5.9 Hz, 2H, CH2), 2.03—2.12(m, 4H, CH2CH2), 1.72—1.69 [m, 6H, C(CH3)2], 1.61(s, 3H, CH3) | 162.61, 150.98, 146.81, 140.86, 131.52, 123.26, 120.44, 118.23, 39.11, 38.08, 25.97, 25.29, 17.32, 16.03 | ||
| 8c | 7.21(d, J=1.1 Hz, 1H, ArH), 6.97(d, J=1.1 Hz, 1H, ArH), 6.42(s, 1H, NH), 5.25—5.39(m, 1H, C═CH), 5.07—5.11(m, 1H, C═CH), 4.03(t, J=6.1 Hz, 2H, CH2), 3.96(s, 3H, OCH3) 2.02—2.12(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.70(s, 3H, CH3), 1.62(s, 3H, CH3) | 164.00, 163.89, 148.94, 146.78, 140.61, 131.50, 123.36, 118.63, 113.63, 106.88, 54.09, 39.13, 37.84, 26.00, 25.31, 17.33, 16.01 | ||
| 8d | 7.26 and 7.27(2s, 2H, ArH), 5.07—5.21(m, 2H, C═CH), 4.14 and 3.76(2d, J=7.1, 6.7 Hz, 2H, CH2), 3.04 and 2.85(2s, 3H, N—CH3), 2.03—2.10(m, 4H, CH2CH2), 1.74 and 1.52(2s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3) | 165.66, 165.20, 150.49, 150.43, 149.07, 141.13, 140.59, 131.48, 131.20, 123.37, 123.11, 120.17, 120.12, 117.69, 117.51, 48.65, 44.19, 39.15, 39.00, 35.64, 32.15, 25.86, 25.80, 25.25, 17.27, 15.82, 15.71 | ||
| 8e | 7.25 and 7.27(2s, 2H, ArH), 5.05—5.22(m, 2H, C═CH), 4.14 and 3.75(2d, J=7.0, 5.8 Hz, 2H, CH2), 3.48—3.55 and 3.14—3.21(2m, 2H, N—CH2), 2.05—2.09(m, 4H, CH2CH2), 1.69(s, 3H, CH3), 1.62 [d, J=4.7 Hz, 6H, C(CH3)2], 1.22 and 1.11(2t, J=7.2, 7.1 Hz, 3H, CH3) | 165.37, 150.55, 150.42, 149.45, 149.33, 140.17, 131.52, 131.21, 123.40, 123.15, 120.04, 119.72, 118.41, 118.28, 46.25, 42.28, 41.29, 39.70, 39.13, 39.00, 25.80, 25.26, 17.29, 15.83, 15.71, 13.55, 12.08 | ||
| 8f | 7.22(d, J=2.0 Hz, 2H, ArH), 5.07—5.30(m, 2H, C═CH), 4.13 and 3.74(2d, J=6.9, 6.7 Hz, 2H, CH2), 3.42 and 3.07(2t, J=7.6, 7.5 Hz, 2H, N—CH2), 2.04—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.48(s, 2H, CH2), 0.97 and 0.80(2t, J=7.4 Hz, 3H, CH3) | 165.64, 150.51, 150.40, 149.45, 140.20, 140.12, 131.48, 131.18, 123.40, 123.14, 119.98, 119.91, 118.39, 118.23, 49.23, 46.58, 46.31, 41.62, 39.11, 38.97, 25.79, 25.25, 21.26, 20.10, 17.27, 15.82, 15.67, 10.97, 10.65 | ||
| 8g | 7.23(s, 2H, ArH), 5.06—5.23(m, 2H, C═CH), 4.13 and 3.74(2d, J=6.8, 6.4 Hz, 2H, CH2), 3.45 and 3.09(2t, J=7.6 Hz, 2H, N—CH2), 2.04—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.48(s, 2H, CH2), 0.97 and 0.80(2t, J=7.4 Hz, 3H, CH3) | 166.14, 165.95, 150.54, 150.46, 149.64, 140.27, 140.18, 131.55, 131.25, 123.39, 123.13, 120.24, 119.88, 118.25, 188.03, 55.00, 51.34, 46.89, 41.74, 39.13, 38.98, 26.39, 26.23, 25.88, 25.79, 25.29, 19.79, 19.43, 17.32, 15.90, 15.69 | ||
| 8h | 7.22(d, J=2.0 Hz, 2H, ArH), 5.06—5.23(m, 2H, C═CH), 4.13 and 3.74(2d, J=6.8, 6.6 Hz, 2H, CH2), 3.44 and 3.08(2t, J=7.7, 7.6 Hz, 2H, N—CH2), 2.04—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.12—1.58(m, 6H, CH2CH2CH2), 0.83—0.95(m, 3H, CH3) | 165.53, 150.51, 150.40, 149.47, 140.12, 131.48, 131.18, 123.39, 123.14, 119.98, 119.88, 118.40, 118.25, 47.60, 46.53, 44.71, 41.65, 39.12, 38.98, 28.71, 28.22, 27.64, 26.49, 25.80, 25.24, 21.98, 21.68, 17.27, 15.85, 15.69, 13.54, 13.37 | ||
| 8i | 7.23(s, 2H, ArH), 5.05—5.23(m, 2H, C═CH), 4.13 and 3.74(2d, J=7.1, 6.5 Hz, 2H, CH2), 3.44 and 3.08(2t, J=7.7, 7.1 Hz, 2H, N—CH2), 2.06—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.17—1.57(m, 8H, CH2CH2CH2CH2), 0.84—0.91(m, 3H, CH3) | 165.51, 150.50, 150.39, 149.47, 140.17, 140.09, 131.46, 131.16, 123.39, 123.14, 119.98, 119.89, 118.41, 118.26, 47.60, 46.53, 44.73, 41.64, 39.12, 38.97, 31.08, 30.75, 27.92, 26.77, 26.23, 25.80, 25.74, 25.24, 22.12, 21.97, 17.26, 15.84, 15.68, 13.56, 13.44 | ||
| 8j | 7.21(s, 2H, ArH), 5.01—5.24(m, 2H, C═CH), 4.64—4.72 and 4.00—4.02(2m, 2H, CH2), 3.68—3.79(m, 2H, CH2), 2.01—2.11(m, 4H, CH2CH2), 1.73(s, 3H, CH3), 1.68(s, 3H, CH3), 1.61(s, 3H, CH3), 1.34(s, 1H, CH), 1.27 and 1.18[2d, J=6.8, 6.5 Hz, 6H, C(CH3)2] | 165.97, 165.22, 150.56, 150.24, 149.94, 138.24, 137.04, 131.41, 131.00, 123.57, 123.27, 120.78, 119.92, 119.46, 50.13, 46.37, 42.72, 38.88, 38.38, 25.81, 25.24, 20.88, 19.72, 17.28, 15.79, 15.56 | ||
| 8k | 7.19—7.21(m, 2H, ArH), 5.03—5.23(m, 2H, C═CH), 4.14 and 3.75(2d, J=7.0, 6.4 Hz, 2H, CH2), 3.31 and 2.95(2d, J=7.6, 7.5 Hz, 2H, N—CH2), 2.04—2.09(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.45(s, 1H, CH), 0.97 and 0.81[2d, J=6.7 Hz, 6H,(CH3)2] | 165.58, 150.54, 150.43, 149.46, 140.16, 131.53, 131.22, 123.40, 123.14, 119.98, 119.89, 118.38, 118.23, 47.39, 46.54, 44.47, 41.66, 39.14, 38.99, 30.11, 28.94, 25.81, 25.26, 19.82, 19.33, 17.29, 15.88, 15.71, 13.42, 13.15 | ||
| 8l | 7.19(s, 2H, ArH), 5.05—5.08(m, 2H, C═CH), 3.77(d, J=5.6 Hz, 2H, CH2), 2.01—2.11(m, 4H, CH2CH2), 1.69(s, 3H, CH3), 1.62(s, 3H, CH3), 1.52[s, 9H, C(CH3)3], 1.34(s, 3H, CH3) | 167.08, 151.36, 150.28, 137.39, 131.44, 123.35, 121.75, 119.50, 57.71, 45.35, 38.81, 28.06, 28.01, 25.77, 25.30, 17.35, 15.66 | ||
| 8m | 7.21(s, 2H, ArH), 5.02—5.15(m, 2H, C═CH), 4.02 and 3.69(2d, J=5.4, 5.9 Hz, 2H, CH2), 4.34 and 3.23(2t, J=14.1, 12.5 Hz, 2H, N—CH2), 2.00—2.17(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.68(s, 3H, CH3), 1.61(s, 3H, CH3), 1.73—1.85 and 1.05—1.57(2m, 10H, CH2CH2CH2CH2CH2) | 166.07, 165.38, 150.60, 150.22, 149.99, 137.98, 136.96, 131.44, 131.02, 123.58, 123.28, 120.97, 119.93, 119.45, 58.71, 54.37, 53.13, 43.10, 39.49, 38.86, 30.17, 25.86, 25.43, 25.24, 17.30, 15.86, 15.58 | ||
| 8n | 7.24—7.30(m, 4H, ArH), 7.01—7.08(m, 2H, ArH), 7.02(d, J=6.9 Hz, 1H, ArH), 5.28—5.33(m, 1H, C═CH), 5.01—5.03(m, 1H, C═CH), 4.48(d, J=7.3 Hz, 2H, CH2), 1.98—2.07(m, 4H, CH2CH2), 1.68(s, 3H, CH3), 1.57(t, J=2.6 Hz, 3H, CH3), 1.50(s, 3H, CH3) | 164.52, 149.94, 148.83, 141.01, 140.73, 131.22, 129.25, 127.74, 127.67, 123.46, 121.54, 117.63, 47.63, 39.14, 25.91, 25.32, 17.32, 15.75 | ||
| 8o | 7.24—7.41(m, 7H, ArH), 5.06—5.30(m, 2H, C═CH), 4.70 and 4.37(2s, 2H, CH2), 4.11 and 3.67(2d, J=6.8, 6.5 Hz, 2H, CH2), 2.04—2.11(m, 4H, CH2CH2), 1.70(s, 3H, CH3), 1.63(s, 3H, CH3), 1.38(s, 3H, CH3) | 166.07, 150.59, 149.03, 141.27, 140.69, 135.99, 135.30, 131.60, 131.31, 128.66, 128.43, 128.01, 127.67, 127.46, 126.35, 123.52, 123.28, 120.06, 117.98, 117.61, 53.17, 51.03, 47.25, 45.83, 41.93, 39.04, 25.83, 25.37, 17.41, 15.82 | ||
Table 3 1H NMR and 13C NMR data of target compounds 7 and 8*
| Compd. | 1H NMR(300 MHz, CDCl3), δ | 13C NMR(75 MHz, CDCl3), δ | ||
|---|---|---|---|---|
| 7a | 8.71—8.73(m, 2H, ArH), 7.80—7.82(m, 2H, ArH), 5.37—5.42(m, 1H, C═CH), 5.03—5.05(m, 1H, C═CH), 4.83(d, J=7.1 Hz, 2H, CH2), 2.00—2.12(m, 4H, CH2CH2), 1.74(s, 3H, CH3), 1.63(s, 3H, CH3), 1.56(s, 3H, CH3) | 164.62, 150.06, 142.86, 137.35, 131.42, 123.24, 122.48, 117.36, 62.18, 39.15, 25.84, 25.22, 17.25, 16.13 | ||
| 7b | 7.80—7.81(m, 2H, ArH), 5.41—5.46(m, 1H, C═CH), 5.06—5.09(m, 1H, C═CH), 4.88(d, J=7.3 Hz, 2H, CH2), 2.07—2.16(m, 4H, CH2CH2), 1.68—1.77 [m, 6H, C(CH3)2], 1.61(s, 3H, CH3) | 162.24, 151.00, 143.75, 142.53, 131.51, 123.17, 122.24, 116.80, 62.94, 39.13, 25.83, 25.25, 17.29, 16.19 | ||
| 7c | 7.44(d, J=1.1 Hz, 1H, ArH), 7.23(d, J=1.1 Hz, 1H, ArH), 5.43—5.48(m, 1H, C═CH), 5.07—5.12(m, 1H, C═CH), 4.86(d, J=7.2 Hz, 2H, CH2), 3.97(s, 3H, OCH3), 2.05—2.17(m, 4H, CH2CH2), 1.78(s, 3H, CH3), 1.69(s, 3H, CH3), 1.62(s, 3H, CH3) | 163.99, 163.47, 148.80, 143.14, 142.46, 131.53, 123.24, 117.19, 115.35, 109.30, 62.42, 54.07, 39.15, 25.87, 25.27, 17.30, 16.18 | ||
| 7d | 8.53(dd, J1=5.1 Hz, J2=0.7 Hz, 1H, ArH), 7.89(dd, J1=1.3 Hz, J2=0.8 Hz, 1H, ArH), 7.78(dd, J1=5.1 Hz, J2=1.4 Hz, 1H, ArH), 5.42—5.48(m, 1H, C═CH), 5.06—5.11(m, 1H, C═CH), 4.88(d, J=7.2 Hz, 2H, CH2), 2.07—2.16(m, 4H, CH2CH2), 1.78(s, 3H, CH3), 1.68(s, 3H, CH3), 1.61(s, 3H, CH3) | 163.23, 151.93, 149.98, 143.18, 140.23, 131.35, 123.54, 123.18, 121.19, 117.08, 62.47, 39.08, 25.79, 25.19, 17.22, 16.10 | ||
| 7e | 8.29(dd, J1=5.3 Hz, J2=0.6 Hz, 1H, ArH), 7.43(dd, J1=5.3 Hz, J2=1.4 Hz, 1H, ArH), 7.34(d, J=0.4 Hz, 1H, ArH), 5.44—5.49(m, 1H, C═CH), 5.08—5.13(m, 1H, C═CH), 4.87(d, J=7.1 Hz, 2H, CH2), 3.98(s, 3H, OCH3), 2.06—2.17(m, 4H, CH2CH2), 1.79(s, 3H, CH3), 1.70(s, 3H, CH3), 1.62(s, 3H, CH3) | 164.68, 164.47, 147.24, 142.79, 140.20, 131.53, 123.29, 117.45, 115.41, 110.87, 62.12, 53.42, 39.17, 25.91, 25.29, 17.32, 16.20 | ||
| 8a | 8.71—8.73(m, 2H, ArH), 7.60—7.62(m, 2H, ArH), 6.28(brs, 1H, NH), 5.26—5.31(m, 1H, C═CH), 5.06—5.10(m, 1H, C═CH), 4.04—4.13(m, 2H, CH2), 2.01—2.11(m, 4H, CH2CH2), 1.68—1.72 [m, 6H, C(CH3)2], 1.60(s, 3H, CH3) | 165.09, 149.63, 141.58, 139.27, 131.16, 123.31, 120.87, 119.19, 39.03, 37.76, 25.92, 25.18, 17.19, 15.82 | ||
| 8b | 7.57(s, 2H, ArH), 6.08(brs, 1H, NH), 5.24—5.29(m, 1H, C═CH), 5.06—5.08(m, 1H, C═CH), 4.05(t, J=5.9 Hz, 2H, CH2), 2.03—2.12(m, 4H, CH2CH2), 1.72—1.69 [m, 6H, C(CH3)2], 1.61(s, 3H, CH3) | 162.61, 150.98, 146.81, 140.86, 131.52, 123.26, 120.44, 118.23, 39.11, 38.08, 25.97, 25.29, 17.32, 16.03 | ||
| 8c | 7.21(d, J=1.1 Hz, 1H, ArH), 6.97(d, J=1.1 Hz, 1H, ArH), 6.42(s, 1H, NH), 5.25—5.39(m, 1H, C═CH), 5.07—5.11(m, 1H, C═CH), 4.03(t, J=6.1 Hz, 2H, CH2), 3.96(s, 3H, OCH3) 2.02—2.12(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.70(s, 3H, CH3), 1.62(s, 3H, CH3) | 164.00, 163.89, 148.94, 146.78, 140.61, 131.50, 123.36, 118.63, 113.63, 106.88, 54.09, 39.13, 37.84, 26.00, 25.31, 17.33, 16.01 | ||
| 8d | 7.26 and 7.27(2s, 2H, ArH), 5.07—5.21(m, 2H, C═CH), 4.14 and 3.76(2d, J=7.1, 6.7 Hz, 2H, CH2), 3.04 and 2.85(2s, 3H, N—CH3), 2.03—2.10(m, 4H, CH2CH2), 1.74 and 1.52(2s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3) | 165.66, 165.20, 150.49, 150.43, 149.07, 141.13, 140.59, 131.48, 131.20, 123.37, 123.11, 120.17, 120.12, 117.69, 117.51, 48.65, 44.19, 39.15, 39.00, 35.64, 32.15, 25.86, 25.80, 25.25, 17.27, 15.82, 15.71 | ||
| 8e | 7.25 and 7.27(2s, 2H, ArH), 5.05—5.22(m, 2H, C═CH), 4.14 and 3.75(2d, J=7.0, 5.8 Hz, 2H, CH2), 3.48—3.55 and 3.14—3.21(2m, 2H, N—CH2), 2.05—2.09(m, 4H, CH2CH2), 1.69(s, 3H, CH3), 1.62 [d, J=4.7 Hz, 6H, C(CH3)2], 1.22 and 1.11(2t, J=7.2, 7.1 Hz, 3H, CH3) | 165.37, 150.55, 150.42, 149.45, 149.33, 140.17, 131.52, 131.21, 123.40, 123.15, 120.04, 119.72, 118.41, 118.28, 46.25, 42.28, 41.29, 39.70, 39.13, 39.00, 25.80, 25.26, 17.29, 15.83, 15.71, 13.55, 12.08 | ||
| 8f | 7.22(d, J=2.0 Hz, 2H, ArH), 5.07—5.30(m, 2H, C═CH), 4.13 and 3.74(2d, J=6.9, 6.7 Hz, 2H, CH2), 3.42 and 3.07(2t, J=7.6, 7.5 Hz, 2H, N—CH2), 2.04—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.48(s, 2H, CH2), 0.97 and 0.80(2t, J=7.4 Hz, 3H, CH3) | 165.64, 150.51, 150.40, 149.45, 140.20, 140.12, 131.48, 131.18, 123.40, 123.14, 119.98, 119.91, 118.39, 118.23, 49.23, 46.58, 46.31, 41.62, 39.11, 38.97, 25.79, 25.25, 21.26, 20.10, 17.27, 15.82, 15.67, 10.97, 10.65 | ||
| 8g | 7.23(s, 2H, ArH), 5.06—5.23(m, 2H, C═CH), 4.13 and 3.74(2d, J=6.8, 6.4 Hz, 2H, CH2), 3.45 and 3.09(2t, J=7.6 Hz, 2H, N—CH2), 2.04—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.48(s, 2H, CH2), 0.97 and 0.80(2t, J=7.4 Hz, 3H, CH3) | 166.14, 165.95, 150.54, 150.46, 149.64, 140.27, 140.18, 131.55, 131.25, 123.39, 123.13, 120.24, 119.88, 118.25, 188.03, 55.00, 51.34, 46.89, 41.74, 39.13, 38.98, 26.39, 26.23, 25.88, 25.79, 25.29, 19.79, 19.43, 17.32, 15.90, 15.69 | ||
| 8h | 7.22(d, J=2.0 Hz, 2H, ArH), 5.06—5.23(m, 2H, C═CH), 4.13 and 3.74(2d, J=6.8, 6.6 Hz, 2H, CH2), 3.44 and 3.08(2t, J=7.7, 7.6 Hz, 2H, N—CH2), 2.04—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.12—1.58(m, 6H, CH2CH2CH2), 0.83—0.95(m, 3H, CH3) | 165.53, 150.51, 150.40, 149.47, 140.12, 131.48, 131.18, 123.39, 123.14, 119.98, 119.88, 118.40, 118.25, 47.60, 46.53, 44.71, 41.65, 39.12, 38.98, 28.71, 28.22, 27.64, 26.49, 25.80, 25.24, 21.98, 21.68, 17.27, 15.85, 15.69, 13.54, 13.37 | ||
| 8i | 7.23(s, 2H, ArH), 5.05—5.23(m, 2H, C═CH), 4.13 and 3.74(2d, J=7.1, 6.5 Hz, 2H, CH2), 3.44 and 3.08(2t, J=7.7, 7.1 Hz, 2H, N—CH2), 2.06—2.11(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.17—1.57(m, 8H, CH2CH2CH2CH2), 0.84—0.91(m, 3H, CH3) | 165.51, 150.50, 150.39, 149.47, 140.17, 140.09, 131.46, 131.16, 123.39, 123.14, 119.98, 119.89, 118.41, 118.26, 47.60, 46.53, 44.73, 41.64, 39.12, 38.97, 31.08, 30.75, 27.92, 26.77, 26.23, 25.80, 25.74, 25.24, 22.12, 21.97, 17.26, 15.84, 15.68, 13.56, 13.44 | ||
| 8j | 7.21(s, 2H, ArH), 5.01—5.24(m, 2H, C═CH), 4.64—4.72 and 4.00—4.02(2m, 2H, CH2), 3.68—3.79(m, 2H, CH2), 2.01—2.11(m, 4H, CH2CH2), 1.73(s, 3H, CH3), 1.68(s, 3H, CH3), 1.61(s, 3H, CH3), 1.34(s, 1H, CH), 1.27 and 1.18[2d, J=6.8, 6.5 Hz, 6H, C(CH3)2] | 165.97, 165.22, 150.56, 150.24, 149.94, 138.24, 137.04, 131.41, 131.00, 123.57, 123.27, 120.78, 119.92, 119.46, 50.13, 46.37, 42.72, 38.88, 38.38, 25.81, 25.24, 20.88, 19.72, 17.28, 15.79, 15.56 | ||
| 8k | 7.19—7.21(m, 2H, ArH), 5.03—5.23(m, 2H, C═CH), 4.14 and 3.75(2d, J=7.0, 6.4 Hz, 2H, CH2), 3.31 and 2.95(2d, J=7.6, 7.5 Hz, 2H, N—CH2), 2.04—2.09(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.69(s, 3H, CH3), 1.61(s, 3H, CH3), 1.45(s, 1H, CH), 0.97 and 0.81[2d, J=6.7 Hz, 6H,(CH3)2] | 165.58, 150.54, 150.43, 149.46, 140.16, 131.53, 131.22, 123.40, 123.14, 119.98, 119.89, 118.38, 118.23, 47.39, 46.54, 44.47, 41.66, 39.14, 38.99, 30.11, 28.94, 25.81, 25.26, 19.82, 19.33, 17.29, 15.88, 15.71, 13.42, 13.15 | ||
| 8l | 7.19(s, 2H, ArH), 5.05—5.08(m, 2H, C═CH), 3.77(d, J=5.6 Hz, 2H, CH2), 2.01—2.11(m, 4H, CH2CH2), 1.69(s, 3H, CH3), 1.62(s, 3H, CH3), 1.52[s, 9H, C(CH3)3], 1.34(s, 3H, CH3) | 167.08, 151.36, 150.28, 137.39, 131.44, 123.35, 121.75, 119.50, 57.71, 45.35, 38.81, 28.06, 28.01, 25.77, 25.30, 17.35, 15.66 | ||
| 8m | 7.21(s, 2H, ArH), 5.02—5.15(m, 2H, C═CH), 4.02 and 3.69(2d, J=5.4, 5.9 Hz, 2H, CH2), 4.34 and 3.23(2t, J=14.1, 12.5 Hz, 2H, N—CH2), 2.00—2.17(m, 4H, CH2CH2), 1.72(s, 3H, CH3), 1.68(s, 3H, CH3), 1.61(s, 3H, CH3), 1.73—1.85 and 1.05—1.57(2m, 10H, CH2CH2CH2CH2CH2) | 166.07, 165.38, 150.60, 150.22, 149.99, 137.98, 136.96, 131.44, 131.02, 123.58, 123.28, 120.97, 119.93, 119.45, 58.71, 54.37, 53.13, 43.10, 39.49, 38.86, 30.17, 25.86, 25.43, 25.24, 17.30, 15.86, 15.58 | ||
| 8n | 7.24—7.30(m, 4H, ArH), 7.01—7.08(m, 2H, ArH), 7.02(d, J=6.9 Hz, 1H, ArH), 5.28—5.33(m, 1H, C═CH), 5.01—5.03(m, 1H, C═CH), 4.48(d, J=7.3 Hz, 2H, CH2), 1.98—2.07(m, 4H, CH2CH2), 1.68(s, 3H, CH3), 1.57(t, J=2.6 Hz, 3H, CH3), 1.50(s, 3H, CH3) | 164.52, 149.94, 148.83, 141.01, 140.73, 131.22, 129.25, 127.74, 127.67, 123.46, 121.54, 117.63, 47.63, 39.14, 25.91, 25.32, 17.32, 15.75 | ||
| 8o | 7.24—7.41(m, 7H, ArH), 5.06—5.30(m, 2H, C═CH), 4.70 and 4.37(2s, 2H, CH2), 4.11 and 3.67(2d, J=6.8, 6.5 Hz, 2H, CH2), 2.04—2.11(m, 4H, CH2CH2), 1.70(s, 3H, CH3), 1.63(s, 3H, CH3), 1.38(s, 3H, CH3) | 166.07, 150.59, 149.03, 141.27, 140.69, 135.99, 135.30, 131.60, 131.31, 128.66, 128.43, 128.01, 127.67, 127.46, 126.35, 123.52, 123.28, 120.06, 117.98, 117.61, 53.17, 51.03, 47.25, 45.83, 41.93, 39.04, 25.83, 25.37, 17.41, 15.82 | ||
| Compd. | Repellent activity(%) | Compd. | Repellent activity(%) | Compd. | Repellent activity(%) |
|---|---|---|---|---|---|
| 7a | 41.9±1.6 | 8d | 33.1±4.5 | 8l | 36.2±2.6 |
| 7b | 59.0±1.6 | 8e | 47.4±2.4 | 8m | 50.7±3.5 |
| 7c | 51.9±3.2 | 8f | 62.0±1.9 | 8n | 61.0±2.7 |
| 7d | 62.6±2.6 | 8g | 56.2±3.6 | 8o | 56.1±0.9 |
| 7e | 21.0±1.8 | 8h | 40.4±2.2 | EBF | 90.6±1.0 |
| 8a | 31.7±2.7 | 8i | 43.6±3.1 | 2b | 0.0±0.0 |
| 8b | 27.7±0.7 | 8j | 47.8±2.3 | 2d | 0.0±0.0 |
| 8c | 25.5±2.8 | 8k | 44.7±3.2 | 3 | 3.5±3.1 |
Table 4 Repellent activity of the target compounds against Myzus persicae(5 μg)
| Compd. | Repellent activity(%) | Compd. | Repellent activity(%) | Compd. | Repellent activity(%) |
|---|---|---|---|---|---|
| 7a | 41.9±1.6 | 8d | 33.1±4.5 | 8l | 36.2±2.6 |
| 7b | 59.0±1.6 | 8e | 47.4±2.4 | 8m | 50.7±3.5 |
| 7c | 51.9±3.2 | 8f | 62.0±1.9 | 8n | 61.0±2.7 |
| 7d | 62.6±2.6 | 8g | 56.2±3.6 | 8o | 56.1±0.9 |
| 7e | 21.0±1.8 | 8h | 40.4±2.2 | EBF | 90.6±1.0 |
| 8a | 31.7±2.7 | 8i | 43.6±3.1 | 2b | 0.0±0.0 |
| 8b | 27.7±0.7 | 8j | 47.8±2.3 | 2d | 0.0±0.0 |
| 8c | 25.5±2.8 | 8k | 44.7±3.2 | 3 | 3.5±3.1 |
| Compd. | Insecticidal activity(%) | Compd. | Insecticidal activity(%) | Compd. | Insecticidal activity(%) |
|---|---|---|---|---|---|
| 7a | 23.5±3.7 | 8d | 70.2±0.3 | 8l | 42.9±4.1 |
| 7b | 63.2±1.1 | 8e | 57.0±4.2 | 8m | 23.8±3.3 |
| 7c | 19.9±1.2 | 8f | 51.1±1.5 | 8n | 46.9±5.3 |
| 7d | 21.3±2.3 | 8g | 30.8±4.6 | 8o | 36.3±4.0 |
| 7e | 30.5±5.6 | 8h | 19.9±2.5 | EBF | 40.0±1.1 |
| 8a | 73.6±3.3 | 8i | 36.8±4.8 | Pymetrozine | 80.7±3.8 |
| 8b | 81.1±5.2 | 8j | 20.1±5.7 | ||
| 8c | 60.3±1.3 | 8k | 30.2±5.1 |
Table 5 Insecticidal activity of the target compounds against Myzus persicae(300 μg/mL)
| Compd. | Insecticidal activity(%) | Compd. | Insecticidal activity(%) | Compd. | Insecticidal activity(%) |
|---|---|---|---|---|---|
| 7a | 23.5±3.7 | 8d | 70.2±0.3 | 8l | 42.9±4.1 |
| 7b | 63.2±1.1 | 8e | 57.0±4.2 | 8m | 23.8±3.3 |
| 7c | 19.9±1.2 | 8f | 51.1±1.5 | 8n | 46.9±5.3 |
| 7d | 21.3±2.3 | 8g | 30.8±4.6 | 8o | 36.3±4.0 |
| 7e | 30.5±5.6 | 8h | 19.9±2.5 | EBF | 40.0±1.1 |
| 8a | 73.6±3.3 | 8i | 36.8±4.8 | Pymetrozine | 80.7±3.8 |
| 8b | 81.1±5.2 | 8j | 20.1±5.7 | ||
| 8c | 60.3±1.3 | 8k | 30.2±5.1 |
| [1] | Yang, Y. , Liu Y., X. , Song H., J. , Li Y., Q. , Wang Q., M. , Bioorg. Med. Chem., 2016, 24( 3), 391- 402 |
| [2] | 杨新玲, 黄文耀, 凌云, 阚伟, 方宇凌, 张钟宁. 高等学校化学学报, 2004, 25( 9), 1657- 1661 |
| Yang X., L. , Huang W., Y. , Ling, Y. , Kan, W. , Fang Y., L. , Zhang Z., N. , Chem. J. Chinese Universities, 2004, 25( 9), 1657- 1661 ( | |
| [3] | David, P. , Environ. Dev. Sustain., 2005, 7( 2), 229- 252 |
| [4] | 黄文耀, 杨新玲, 张钟宁. 化学通报, 2002, 3, 157- 161 |
| Huang W., Y. , Yang X., L. , Zhang Z., N. , Chemistry Bulletin, 2002, 3, 157- 161 ( | |
| [5] | 张钟宁, 刘珣, 梅雪琴. 动物学集刊, 1993, 10, 1- 3 |
| Zhang Z., N. , Liu, X. , Mei X., Q. , Animal Bulletin, 1993, 10, 1- 3 ( | |
| [6] | 闫凤鸣, 陈巨莲, 汤清波. 植物保护, 2013, 39( 5), 9- 15 |
| Yan F., M. , Chen J., L. , Tang Q., B. , Plant Protection, 2013, 39( 5), 9- 15 ( | |
| [7] | Kislow C., J. , Edward L., J. , Nature, 1972, 235( 5333), 108- 109 |
| [8] | Bowers W., S. , Nault L., R. , Webb R., E. , Dutky S., R. , Science, 1972, 175( 4026), 1121- 1122 |
| [9] | Edwards L., J. , Siddall J., B. , Dunham L., L. , Uden, P. , Kislow C., J. , Nature, 1973, 241( 5385), 126- 127 |
| [10] | Van O. A., M. , Gut, J. , Harrewijn, P. , Piron P. G., M. , Acta Phytopathol. Entomol. Hung., 1990, 25( 1-4), 331- 342 |
| [11] | CuiL., L. , Dong, J. , Francis, F. , Liu Y., J. , Heuskin, S. , Lognay, G. , Chen J., L. , Bragard, C. , Tooker J., F. , Liu, Y. , Crop Protection, 2012, 35, 91- 96 |
| [12] | Nishino, C. , Bowers W., S. , Montgomery M., E. , Nault L., R. , Appl. Entomol. Zool., 1976, 11( 4), 340- 343 |
| [13] | Dawson G., W. , Gibson R., W. , Griffiths D., C. , Pickett J., A. , Rice A., D. , Woodcock C., M. , J. Chem. Ecol., 1982, 8( 11), 1377- 1388 |
| [14] | 李正名, 王天生, 么恩云, 陈学仁, 朱兰蕙, 王素华. 化学学报, 1987, 45, 1124- 1128 |
| Li Z., M. , Wang T., S. , Me E., Y. , Chen X., R. , Zhu L., H. , Wang S., H. , Acta Chemical Sinica, 1987, 45, 1124- 1128 ( | |
| [15] | 张钟宁, 刘珣, Pickett John. 昆虫学报, 1988, 31( 4), 435- 438 |
| Zhang Z., N. , Liu, X. , Pickett, J. , Acta Entomol. Sinica, 1988, 31( 4), 435- 438 ( | |
| [16] | 孙玉凤, 李永强, 凌云, 宇红莲, 杨绍祥, 杨新玲. 有机化学, 2011, 31( 9), 1425- 1432 |
| Sun Y., F. , Li Y., Q. , Ling, Y. , Yu H., L. , Yang S., X. , Yang X., L. , Chin. J. Org. Chem., 2011, 31( 9), 1425- 1432 ( | |
| [17] | 秦耀果, 曲焱焱, 张景朋, 谭晓庆, 宋丽芳, 李文浩, 宋敦伦, 杨新玲. 有机化学, 2015, 35( 2), 455- 461 |
| Qin Y., G. , Qu Y., Y. , Zhang J., P. , Tan X., Q. , Song L., F. , Li W., H. , Song D., L. , Yang X., L. , Chin. J. Org. Chem., 2015, 35( 2), 455- 461 ( | |
| [18] | Song B. A., Jin L. H., New Heterocyclic Pesticide, Chemical Industry Press, Beijing, 2010, 118— 128 |
| ( 宋宝安, 金林红. 新杂环农药, 北京: 化学工业出版社, 2010, 118— 128) | |
| [19] | 张燕, 王宝雷, 詹益周, 张丽媛, 李永红. 李正名. 高等学校化学学报, 2016, 37( 6), 1100- 1107 |
| Zhang, Y. , Wang B., L. , Zhan Y., Z. , Zhang L., Y. , Li Y., H. , Li Z., M. , Chem. J. Chinese Universities, 2016, 37( 6), 1100- 1107 ( | |
| [20] | Gao, Y. , Wang B., W. , Gao, S. , Zhang R., H. , Yang C., Y. , Sun, Z. , Liu Z., H. , Chem. Res. Chinese Universities, 2016, 32( 4), 594- 599 |
| [21] | Tian Z., Z. , Zhang, D. , Guo, B. , Tian, G. , Liu X., X. , Yue H., J. , Chem. Res. Chinese Universities, 2015, 31( 2), 249- 252 |
| [22] | 王华森, 怀其勇. 天然产物研究与开发, 2013, 25( 2), 237- 240 |
| Wang H., S. , Huai Q., Y. , Nat. Prod. Res. Dev., 2013, 25( 2), 237- 240 ( | |
| [23] | Wild, N. , Groth, U. , Eur. J. Org. Chem., 2003, 2003( 22), 4445- 4449 |
| [24] | Peter, W. , John P., M. , Org. Lett., 2008, 10( 19), 4383- 4386 |
| [25] | Maciej, A. , Srinivasa R., A. , Rajarathnam E., R. , Tetrahedron, 2002, 58( 34), 6951- 6963 |
| [26] | 孙亮. EBF类似物CAU-1204的合成工艺及结构优化研究. 北京: 中国农业大学, 2013) |
| Sun, L. , Synthesis Process and Structure Optimization of EBF Analogue CAU- 1204, China Agricultural University, Beijing, 2013 ( | |
| [27] | Ravikumar P., C. , Yao L., H. , Fleming F., F. , J. Org. Chem., 2009, 74( 19), 7294- 7299 |
| [1] | 赵梦阳, 黄紫洋. HA/CuO/SrCO3梯度复合涂层的制备及体外生物活性[J]. 高等学校化学学报, 2022, 43(2): 20210644. |
| [2] | 魏思敏,王英辉,唐志书,苏瑞,胡锦航,郭惠,李琛,蒋金涛,宋忠兴. 紫外光辐射山茱萸水提液制备纳米银及生物活性[J]. 高等学校化学学报, 2020, 41(6): 1391. |
| [3] | 高其龙, 梁二军, 邢献然, 陈骏. 普鲁士蓝类化合物负热膨胀及机理[J]. 高等学校化学学报, 2020, 41(3): 388. |
| [4] | 成沁雯, 袁波, 朱向东, 张凯, 张兴栋. 不同磺化及碱处理聚醚醚酮的表面化学组成及体外生物活性评价[J]. 高等学校化学学报, 2019, 40(8): 1757. |
| [5] | 阮祥辉, 赵洪菊, 张橙, 陈丽娟, 李普, 王一会, 贺鸣, 薛伟. 含哌嗪酰胺类杨梅素衍生物的合成及生物活性[J]. 高等学校化学学报, 2018, 39(6): 1197. |
| [6] | 李阳, 李志文, 朱俊飞, 刘世会, 何军林. 芘基对在dsDNA中的构建:基于8-氮-7-去氮-2'-脱氧腺苷的7位取代及连接臂对荧光性质的影响[J]. 高等学校化学学报, 2018, 39(10): 2206. |
| [7] | 段永斌, 殷燕, 孟凡丽, 赵连花, 刘玉坤, 袁哲, 冯阳波. 基于分子对接和自由能计算的高活性苯并噻唑类ROCK抑制剂的设计、 合成和生物学评价[J]. 高等学校化学学报, 2017, 38(9): 1568. |
| [8] | 石玉军, 方源, 李阳, 陈佳, 李刚, 汪清民, 戴红. 新型含5-芳基异噁唑结构的氰基丙烯酸酯类化合物的合成及生物活性[J]. 高等学校化学学报, 2017, 38(3): 421. |
| [9] | 白信法, 马宣, 解晓霞, 邵明莎, 郭宁宁, 严宁, 姚雷. 微管菌素类似物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2017, 38(1): 47. |
| [10] | 张燕, 王宝雷, 詹益周, 张丽媛, 李永红, 李正名. 新型含取代哌嗪的5-(吡啶-3-基)-1,2,4-三唑Mannich碱和双Mannich碱的合成及生物活性[J]. 高等学校化学学报, 2016, 37(6): 1100. |
| [11] | 徐高飞, 刘艳红, 杨新玲, 王道全, 袁德凯. 含有双氰基环丙烷甲酰胺类化合物的合成及生物活性[J]. 高等学校化学学报, 2016, 37(3): 486. |
| [12] | 王子剑, 孙晓红, 刘源发, 陈邦, 沈生强, 靳如意, 马海霞. 含吡唑环的1,2,4-三唑希夫碱类衍生物的合成及生物活性[J]. 高等学校化学学报, 2015, 36(7): 1315. |
| [13] | 李旭, 蒋建宏, 韩布兴, 谷惠文, 谢兆凤, 陈兰, 肖圣雄, 李传华, 李爱桃, 李霞, 姚飞虹, 王群, 李强国. 邻香草醛缩组氨酸Schiff碱及其镧配合物的合成与生物活性[J]. 高等学校化学学报, 2015, 36(5): 856. |
| [14] | 马永涛, 冯思良, 王晨宏, 周宁, 刘克良. 促性腺激素释放激素类似物-紫杉醇靶向抗肿瘤缀合物的合成及评价[J]. 高等学校化学学报, 2014, 35(9): 1896. |
| [15] | 沈生强, 孙晓红, 刘源发, 陈邦, 靳如意, 马海霞. 含嘧啶环的1,3,4-噁二唑Mannich碱的合成及生物活性[J]. 高等学校化学学报, 2014, 35(7): 1427. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||