

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (9): 1649.doi: 10.7503/cjcu20160145
李林1,2, 李淼3, 柴宝山4, 杨吉春3, 宋玉泉3, 刘长令3( )
)
收稿日期:2016-03-11
出版日期:2016-09-10
发布日期:2016-08-17
作者简介:联系人简介: 刘长令, 男, 博士, 总工程师, 主要从事新农药创制研究. E-mail:
LI Lin1,2, LI Miao3, CHAI Baoshan4, YANG Jichun3, SONG Yuquan3, LIU Changling3,*( )
)
Received:2016-03-11
Online:2016-09-10
Published:2016-08-17
Contact:
LIU Changling
E-mail:liuchangling@vip.163.com
摘要:
利用中间体衍生化方法, 将噻吩环引入到双酰胺类化合物中, 合成了一系列取代噻吩双酰胺类化合物1~3; 目标化合物的结构经核磁共振波谱、 红外光谱及元素分析确认. 生物活性测试结果表明, 化合物1在600 mg/L剂量下对小菜蛾具有良好的杀虫效果, 致死率均为100%, 其中化合物1a和1e在20 mg/L剂量下对小菜蛾的致死率仍达到60%以上; 改变双酰胺结构中的吡唑环得到化合物2和3, 其杀虫活性消失, 说明该类化合物中吡唑环结构对杀虫活性具有关键作用.
中图分类号:
TrendMD:
李林, 李淼, 柴宝山, 杨吉春, 宋玉泉, 刘长令. 取代噻吩双酰胺类化合物的设计、 合成及生物活性. 高等学校化学学报, 2016, 37(9): 1649.
LI Lin, LI Miao, CHAI Baoshan, YANG Jichun, SONG Yuquan, LIU Changling. Design, Synthesis and Biological Activity of Novel Substituted Diamides Derivatives Containing Thiophene Ring. Chem. J. Chinese Universities, 2016, 37(9): 1649.
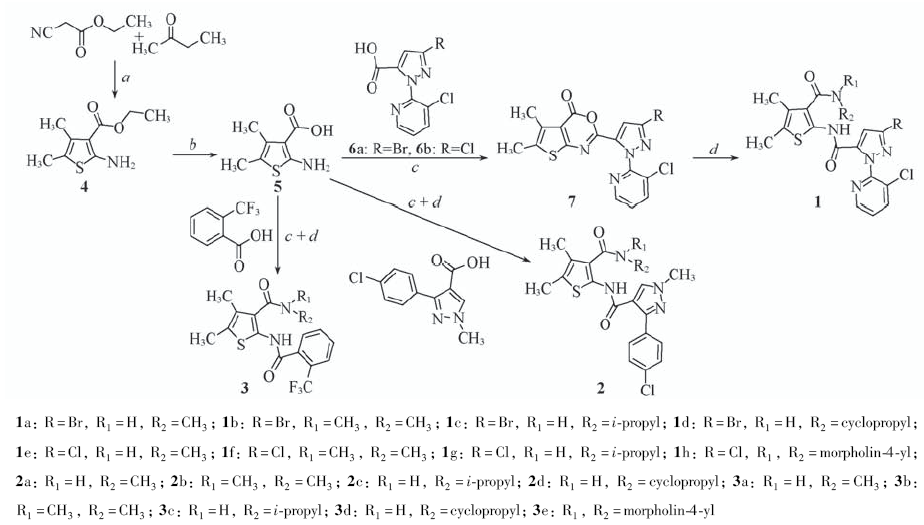
Scheme 1 Synthetic routes of compounds 1—3 Reagents and conditions: a. S, NHEt2, CH3CN, 50 ℃, 1—5 h; b. NaOH, CH3CH2OH/H2O, reflux, 2 h; c. CH3SO2Cl, pyridine, CH3CN, 2 h; d. R1R2NH, CH3CN, 1—6 h.
| Compd. | Yield (%) | m.p./℃ | Elemental analysis(%, calcd.) | IR, | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 1a | 23 | 221—222 | 42.38 (43.56) | 3.44 (3.23) | 15.03 (14.94) | 3460(m, N—H), 2980, 2960(m, C—H), 1620(s, C=O), 1565, 1455(m, aromatic rings), 1450, 1360(m, C—H), 1320(s, C—O), 830, 750, 720(m, Ph-H) |
| 1b | 29 | 238—239 | 45.65 (44.78) | 3.50 (3.55) | 14.34 (14.51) | 3440(m, N—H), 2970, 2940(m, C—H), 1610(s, C=O), 1555, 1440(m, aromatic rings), 1440, 1370(m, C—H), 1310(s, C—O), 810, 770, 730(m, Ph-H) |
| 1c | 21 | 224—225 | 46.524 (45.9) | 3.73 (3.86) | 14.02 (14.10) | 3450(m, N—H), 2980(m, C—H), 1620(s, C=O), 1560, 1440(m, aromatic rings), 1450, 1370(m, C—H), 1330(s, C—O), 820, 760, 700(m, Ph-H) |
| 1d | 25 | 238—239 | 45.43 (46.12) | 3.58 (3.46) | 14.27 (14.15) | 3440(m, N—H), 2950(m, C—H), 1600(s, C=O), 1555, 1435(m, aromatic rings), 1450, 1360(m, C—H), 1320(s, C—O), 800, 770, 720(m, Ph-H) |
| 1e | 32 | 217—218 | 47.36 (48.12) | 3.55 (3.56) | 16.63 (16.51) | 3460(m, N—H), 2975, 2960(m, C—H), 1620(s, C=O), 1560, 1450(m, aromatic rings), 1470, 1370(m, C—H), 1330(s, C—O), 830, 760, 690(m, Ph-H) |
| 1f | 58 | 235—236 | 49.28 (49.32) | 3.89 (3.91) | 15.99 (15.98) | 3440(m, N—H), 2970(m, C—H), 1610(s, C=O), 1565, 1455(m, aromatic rings), 1460, 1350(m, C—H), 1310(s, C—O), 820, 750, 710(m, Ph-H) |
| 1g | 41 | 217—218 | 51.78 (50.45) | 4.16 (4.23) | 15.38 (15.48) | 3430(m, N—H), 2980, 2960(m, C—H), 1630(s, C=O), 1570, 1460(m, aromatic rings), 1470, 1365(m, C—H), 1320(s, C—O), 820, 770, 730(m, Ph-H) |
| 1h | 50 | 257—258 | 51.11 (50.01) | 3.82 (3.99) | 14.50 (14.58) | 3420(m, N—H), 2980, 2960(m, C—H), 1600(s, C=O), 1550, 1440(m, aromatic rings), 1440, 1370(m, C—H), 1300(s, C—O), 800, 760, 740(m, Ph-H) |
| 2a | 25 | 170—171 | 57.84 (56.64) | 4.67 (4.75) | 13.86 (13.91) | 3420(m, N—H), 2980, 2960(m, C—H), 1600(s, C=O), 1550, 1440(m, aromatic rings), 1440, 1370(m, C—H), 1300(s, C—O), 800, 760, 740(m, Ph-H) |
| 2b | 38 | 175—176 | 56.90 (57.62) | 5.21 (5.08) | 13.52 (13.44) | 3440(m, N—H), 2980, 2950(m, C—H), 1620(s, C=O), 1530(m, aromatic rings), 1420, 1350(m, C—H), 1330(s, C—O), 820, 780, 720(m, Ph-H) |
| 2c | 30 | 234—235 | 57.46 (58.53) | 5.44 (5.38) | 13.15 (13.00) | 3430(m, N—H), 2960(m, C—H), 1610(s, C=O), 1540(m, aromatic rings), 1440, 1370(m, C—H), 1300(s, C—O), 800, 760, 740(m, Ph-H) |
| 2d | 40 | 245—246 | 57.69 (58.80) | 5.02 (4.94) | 13.13 (13.06) | 3450(m, N—H), 2980(s, C—H), 1620(s, C=O), 1520(s, aromatic rings), 1450, 1390(m, C—H), 1220, 1200(s, C—O), 840, 760(s, Ph-H) |
| 3a | 52 | 127—128 | 54.63 (53.93) | 4.18 (4.24) | 7.78 (7.86) | 3440(m, N—H), 2970(m, C—H), 1670(s, C=O), 1620, 1560, 1510(s, aromatic rings), 1400, 1310(m, C—H), 1130(s, C—F), 780, 710(m, Ph-H) |
| 3b | 50 | 185—186 | 56.87 (55.13) | 4.57 (4.63) | 7.42 (7.56) | 3430(m, N—H), 2950(m, C—H), 1660(s, C=O), 1610, 1550, 1500(s, aromatic rings), 1410, 1320(m, C—H), 1120(s, C—F), 820, 790, 700(m, Ph-H) |
| 3c | 52 | 147—148 | 55.30 (56.24) | 5.07 (4.98) | 7.35 (7.29) | 3440(m, N—H), 2960(m, C—H), 1670(s, C=O), 1600, 1550(s, aromatic rings), 1420, 1320(m, C—H), 1110(s, C—F), 760, 700(m, Ph-H) |
| 3d | 58 | 151—152 | 56.58 (56.54) | 4.49 (4.48) | 7.34 (7.33) | 3420(m, N—H), 2970(m, C—H), 1650(s, C=O), 1620, 1540(s, aromatic rings), 1430, 1320(m, C—H), 1130(s, C—F), 830, 780, 720(m, Ph-H) |
| 3e | 33 | 187—188 | 55.90 (55.33) | 4.71 (4.64) | 6.68 (6.79) | 3430(m, N—H), 2950(m, C—H), 1660(s, C=O), 1630, 1580, 1520(s, aromatic rings), 1410, 1300(m, C—H), 1120(s, C—F), 800, 770, 710(m, Ph-H) |
Table 1 Yields, melting points, elemental analysis and IR data of compounds 1—3
| Compd. | Yield (%) | m.p./℃ | Elemental analysis(%, calcd.) | IR, | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 1a | 23 | 221—222 | 42.38 (43.56) | 3.44 (3.23) | 15.03 (14.94) | 3460(m, N—H), 2980, 2960(m, C—H), 1620(s, C=O), 1565, 1455(m, aromatic rings), 1450, 1360(m, C—H), 1320(s, C—O), 830, 750, 720(m, Ph-H) |
| 1b | 29 | 238—239 | 45.65 (44.78) | 3.50 (3.55) | 14.34 (14.51) | 3440(m, N—H), 2970, 2940(m, C—H), 1610(s, C=O), 1555, 1440(m, aromatic rings), 1440, 1370(m, C—H), 1310(s, C—O), 810, 770, 730(m, Ph-H) |
| 1c | 21 | 224—225 | 46.524 (45.9) | 3.73 (3.86) | 14.02 (14.10) | 3450(m, N—H), 2980(m, C—H), 1620(s, C=O), 1560, 1440(m, aromatic rings), 1450, 1370(m, C—H), 1330(s, C—O), 820, 760, 700(m, Ph-H) |
| 1d | 25 | 238—239 | 45.43 (46.12) | 3.58 (3.46) | 14.27 (14.15) | 3440(m, N—H), 2950(m, C—H), 1600(s, C=O), 1555, 1435(m, aromatic rings), 1450, 1360(m, C—H), 1320(s, C—O), 800, 770, 720(m, Ph-H) |
| 1e | 32 | 217—218 | 47.36 (48.12) | 3.55 (3.56) | 16.63 (16.51) | 3460(m, N—H), 2975, 2960(m, C—H), 1620(s, C=O), 1560, 1450(m, aromatic rings), 1470, 1370(m, C—H), 1330(s, C—O), 830, 760, 690(m, Ph-H) |
| 1f | 58 | 235—236 | 49.28 (49.32) | 3.89 (3.91) | 15.99 (15.98) | 3440(m, N—H), 2970(m, C—H), 1610(s, C=O), 1565, 1455(m, aromatic rings), 1460, 1350(m, C—H), 1310(s, C—O), 820, 750, 710(m, Ph-H) |
| 1g | 41 | 217—218 | 51.78 (50.45) | 4.16 (4.23) | 15.38 (15.48) | 3430(m, N—H), 2980, 2960(m, C—H), 1630(s, C=O), 1570, 1460(m, aromatic rings), 1470, 1365(m, C—H), 1320(s, C—O), 820, 770, 730(m, Ph-H) |
| 1h | 50 | 257—258 | 51.11 (50.01) | 3.82 (3.99) | 14.50 (14.58) | 3420(m, N—H), 2980, 2960(m, C—H), 1600(s, C=O), 1550, 1440(m, aromatic rings), 1440, 1370(m, C—H), 1300(s, C—O), 800, 760, 740(m, Ph-H) |
| 2a | 25 | 170—171 | 57.84 (56.64) | 4.67 (4.75) | 13.86 (13.91) | 3420(m, N—H), 2980, 2960(m, C—H), 1600(s, C=O), 1550, 1440(m, aromatic rings), 1440, 1370(m, C—H), 1300(s, C—O), 800, 760, 740(m, Ph-H) |
| 2b | 38 | 175—176 | 56.90 (57.62) | 5.21 (5.08) | 13.52 (13.44) | 3440(m, N—H), 2980, 2950(m, C—H), 1620(s, C=O), 1530(m, aromatic rings), 1420, 1350(m, C—H), 1330(s, C—O), 820, 780, 720(m, Ph-H) |
| 2c | 30 | 234—235 | 57.46 (58.53) | 5.44 (5.38) | 13.15 (13.00) | 3430(m, N—H), 2960(m, C—H), 1610(s, C=O), 1540(m, aromatic rings), 1440, 1370(m, C—H), 1300(s, C—O), 800, 760, 740(m, Ph-H) |
| 2d | 40 | 245—246 | 57.69 (58.80) | 5.02 (4.94) | 13.13 (13.06) | 3450(m, N—H), 2980(s, C—H), 1620(s, C=O), 1520(s, aromatic rings), 1450, 1390(m, C—H), 1220, 1200(s, C—O), 840, 760(s, Ph-H) |
| 3a | 52 | 127—128 | 54.63 (53.93) | 4.18 (4.24) | 7.78 (7.86) | 3440(m, N—H), 2970(m, C—H), 1670(s, C=O), 1620, 1560, 1510(s, aromatic rings), 1400, 1310(m, C—H), 1130(s, C—F), 780, 710(m, Ph-H) |
| 3b | 50 | 185—186 | 56.87 (55.13) | 4.57 (4.63) | 7.42 (7.56) | 3430(m, N—H), 2950(m, C—H), 1660(s, C=O), 1610, 1550, 1500(s, aromatic rings), 1410, 1320(m, C—H), 1120(s, C—F), 820, 790, 700(m, Ph-H) |
| 3c | 52 | 147—148 | 55.30 (56.24) | 5.07 (4.98) | 7.35 (7.29) | 3440(m, N—H), 2960(m, C—H), 1670(s, C=O), 1600, 1550(s, aromatic rings), 1420, 1320(m, C—H), 1110(s, C—F), 760, 700(m, Ph-H) |
| 3d | 58 | 151—152 | 56.58 (56.54) | 4.49 (4.48) | 7.34 (7.33) | 3420(m, N—H), 2970(m, C—H), 1650(s, C=O), 1620, 1540(s, aromatic rings), 1430, 1320(m, C—H), 1130(s, C—F), 830, 780, 720(m, Ph-H) |
| 3e | 33 | 187—188 | 55.90 (55.33) | 4.71 (4.64) | 6.68 (6.79) | 3430(m, N—H), 2950(m, C—H), 1660(s, C=O), 1630, 1580, 1520(s, aromatic rings), 1410, 1300(m, C—H), 1120(s, C—F), 800, 770, 710(m, Ph-H) |
| Compd. | 1H NMR(300 MHz), δ |
|---|---|
| 1a | 13.14(s, 1H, NH), 8.49(dd, J=1.5, 4.5 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.5, 1.8 Hz, 1H, pyridin-4-H), 7.42(dd, J=4.5, 8.4 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 6.11(s, 1H, NH), 3.03(d, J=3.0 Hz, 3H, CH3), 2.30(s, 3H, thiophene-5-CH3), 2.25(s, 3H, thiophene-4-CH3) |
| 1b | 11.97(s, 1H, NH), 8.52(dd, J=1.5, 4.8 Hz, 1H, pyridin-6-H), 8.23(dd, J=1.5, 4.8 Hz, 1H, pyridin-4-H), 7.66(dd, J=4.8, 8.4 Hz, 1H, pyridin-5-H), 7.34(s, 1H, pyrazole), 2.87(s, 3H, CH3), 2.79(s, 3H, CH3), 2.21(s, 3H, thiophene-5-CH3), 1.91(s, 3H, thiophene-4-CH3) |
| 1c | 13.11(s, 1H, NH), 8.49(dd, J=1.8, 5.1 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.8, 5.1 Hz, 1H, pyridin-4-H), 7.42(dd, J=5.1, 8.1 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 5.89(br, 1H, NH), 4.28(m, 1H, CH), 2.27(s, 3H, thiophene-5-CH3), 2.23(s, 3H, thiophene-4-CH3), 1.28(d, J=5.1 Hz, 6H, 2CH3) |
| 1d | 11.91(s, 1H, NH), 8.54(dd, J=1.2, 4.8 Hz, 1H, pyridin-6-H), 8.25(dd, J=1.5, 4.5 Hz, 1H, pyridin-4-H), 7.68(dd, J=4.8, 8.1 Hz, 1H, pyridin-5-H), 7.29(s, 1H, pyrazole), 6.98(s, 1H, NH), 2.81(m, 1H, CH), 2.10(s, 3H, thiophene-5-CH3), 2.06(s, 3H, thiophene-4-CH3), 0.70(m, 2H, CH2), 0.55(m, 2H, CH2) |
| 1e | 13.14(s, 1H, NH), 8.49(dd, J=1.5, 5.1 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.2, 5.1 Hz, 1H, pyridin-4-H), 7.42(dd, J=4.8, 8.7 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 6.11(s, 1H, NH), 3.03(d, J=3.3 Hz, 3H, CH3), 2.30(s, 3H, thiophene-5-CH3), 2.25(s, 3H, thiophene-4-CH3) |
| 1f | 10.45(s, 1H, NH), 8.50(dd, J=1.8, 4.5 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.8, 4.8 Hz,1H, pyridin-4-H), 7.42(dd, J=4.5, 8.4 Hz, 1H, pyridin-5-H), 6.99(s, 1H, pyrazole-H), 3.03(s, 6H, 2CH3), 2.23(s, 3H, thiophene-5-CH3), 2.03(s, 3H, thiophene-4-CH3) |
| 1g | 13.11(s, 1H, NH), 8.49(dd, J=1.2, 5.1 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.5, 5.1 Hz, 1H, pyridin-4-H), 7.42(dd, J=5.1, 8.7 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 5.89(br, 1H, NH), 4.28(m, 1H, CH), 2.27(s, 3H, thiophene-5-CH3), 2.23(s, 3H, thiophene-4-CH3), 1.28(d, J=6.9 Hz, 6H, 2CH3) |
| 1h | 10.43(s, 1H, NH), 8.49(dd, J=1.5, 4.8 Hz, 1H, pyridin-6-H), 7.91(dd, J=1.2, 4.8 Hz, 1H, pyridin-4-H), 7.43(dd, J=4.8, 8.4 Hz, 1H, pyridin-5-H), 6.96(s, 1H, pyrazole-H), 3.71(m, 4H, morpholine-2,6-2CH2), 3.54(m, 4H, morpholine-3,5-2CH2), 2.22(s, 3H, thiophene-5-CH3), 2.04(s, 3H, thiophene-4-CH3) |
| 2a | 8.33(s, 1H, pyrazole-H), 7.70(s, 1H, NH), 7.65(d, J=6.6 Hz, 2H, Ph-2,6-2H), 7.47(d, J=6.6 Hz, 2H, Ph-3,5-2H), 3.93(s, 3H, pyrazole-CH3), 2.93(s, 3H, CH3), 2.23(s, 3H, thiophene-5-CH3), 1.92(s, 3H, thiophene-4-CH3) |
| 2b | 8.23(s, 1H, pyrazole-H), 7.71(s, 1H, NH), 7.68(d, J=6.9 Hz, 2H, Ph-2,6-2H), 7.47(d, J=6.9 Hz, 2H, Ph-3,5-2H), 3.93(s, 3H, pyrazole-CH3), 2.83(s, 6H, 2CH3), 2.23(s, 3H, thiophene-5-CH3), 1.92(s, 3H, thiophene-4-CH3) |
| 2c | 12.01(s, 1H, NH), 8.31(s, 1H, pyrazole-H), 7.68(d, J=6.3 Hz, 2H, Ph-2,6-2H), 7.47(d, J=6.3 Hz, 2H, Ph-3,5-2H), 3.96(m, 1H, CH), 3.95(s, 3H, pyrazole-CH3), 2.23(s, 3H, thiophene-5-CH3), 2.15(s, 3H, thiophene-4-CH3), 1.13(s, 3H, CH3), 1.11(s, 3H, CH3) |
| 2d | 8.29(s, 1H, pyrazole-H), 7.71(s, 1H, NH), 7.66(d, J=6.6 Hz, 2H, Ph-2,6-2H), 7.43(d, J=6.6 Hz, 2H, Ph-3,5-2H), 3.93(s, 3H, pyrazole-CH3), 2.32(m, 1H, CH), 2.23(s, 3H, thiophene-5-CH3), 1.92(s, 3H, thiophene-4-CH3), 0.83(m, 4H, 2CH2) |
| 3a | 12.40(s, 1H, NH), 7.77—7.58(m, 4H, Ph), 6.05(s, 1H, NH), 2.95(d, J=3.0 Hz, 3H, CH3), 2.32(s, 6H, thiophene-2CH3) |
| 3b | 9.19(s, 1H, NH), 7.75—7.59(m, 4H, Ph), 3.02(s, 6H, 2CH3), 2.32(s, 3H, thiophene-5-CH3), 2.06(s, 3H, thiophene-4-CH3) |
| 3c | 12.31(s, 1H, NH), 7.76—7.58(m, 4H, Ph), 5.85(br, 1H, NH), 4.19(m, 1H, CH), 2.31(s, 6H, thiophene-2CH3), 1.24(d, J=5.1 Hz, 6H, 2CH3) |
| 3d | 12.39(s, 1H, NH), 7.77—7.58(m, 4H, Ph), 6.19(s, 1H, NH), 2.80(m, 1H, CH), 2.31(s, 3H, thiophene-5-CH3), 2.26(s, 3H, thiophene-4-CH3), 0.85(m, 4H, 2CH2) |
| 3e | 9.26(s, 1H, NH), 7.76—7.64(m, 4H, Ph), 3.78—3.46(m, 8H, morpholine), 2.31(s, 3H, thiophene-5-CH3), 2.07(s, 3H, thiophene-4-CH3) |
Table 2 1H NMR data of compounds 1—3*
| Compd. | 1H NMR(300 MHz), δ |
|---|---|
| 1a | 13.14(s, 1H, NH), 8.49(dd, J=1.5, 4.5 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.5, 1.8 Hz, 1H, pyridin-4-H), 7.42(dd, J=4.5, 8.4 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 6.11(s, 1H, NH), 3.03(d, J=3.0 Hz, 3H, CH3), 2.30(s, 3H, thiophene-5-CH3), 2.25(s, 3H, thiophene-4-CH3) |
| 1b | 11.97(s, 1H, NH), 8.52(dd, J=1.5, 4.8 Hz, 1H, pyridin-6-H), 8.23(dd, J=1.5, 4.8 Hz, 1H, pyridin-4-H), 7.66(dd, J=4.8, 8.4 Hz, 1H, pyridin-5-H), 7.34(s, 1H, pyrazole), 2.87(s, 3H, CH3), 2.79(s, 3H, CH3), 2.21(s, 3H, thiophene-5-CH3), 1.91(s, 3H, thiophene-4-CH3) |
| 1c | 13.11(s, 1H, NH), 8.49(dd, J=1.8, 5.1 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.8, 5.1 Hz, 1H, pyridin-4-H), 7.42(dd, J=5.1, 8.1 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 5.89(br, 1H, NH), 4.28(m, 1H, CH), 2.27(s, 3H, thiophene-5-CH3), 2.23(s, 3H, thiophene-4-CH3), 1.28(d, J=5.1 Hz, 6H, 2CH3) |
| 1d | 11.91(s, 1H, NH), 8.54(dd, J=1.2, 4.8 Hz, 1H, pyridin-6-H), 8.25(dd, J=1.5, 4.5 Hz, 1H, pyridin-4-H), 7.68(dd, J=4.8, 8.1 Hz, 1H, pyridin-5-H), 7.29(s, 1H, pyrazole), 6.98(s, 1H, NH), 2.81(m, 1H, CH), 2.10(s, 3H, thiophene-5-CH3), 2.06(s, 3H, thiophene-4-CH3), 0.70(m, 2H, CH2), 0.55(m, 2H, CH2) |
| 1e | 13.14(s, 1H, NH), 8.49(dd, J=1.5, 5.1 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.2, 5.1 Hz, 1H, pyridin-4-H), 7.42(dd, J=4.8, 8.7 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 6.11(s, 1H, NH), 3.03(d, J=3.3 Hz, 3H, CH3), 2.30(s, 3H, thiophene-5-CH3), 2.25(s, 3H, thiophene-4-CH3) |
| 1f | 10.45(s, 1H, NH), 8.50(dd, J=1.8, 4.5 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.8, 4.8 Hz,1H, pyridin-4-H), 7.42(dd, J=4.5, 8.4 Hz, 1H, pyridin-5-H), 6.99(s, 1H, pyrazole-H), 3.03(s, 6H, 2CH3), 2.23(s, 3H, thiophene-5-CH3), 2.03(s, 3H, thiophene-4-CH3) |
| 1g | 13.11(s, 1H, NH), 8.49(dd, J=1.2, 5.1 Hz, 1H, pyridin-6-H), 7.89(dd, J=1.5, 5.1 Hz, 1H, pyridin-4-H), 7.42(dd, J=5.1, 8.7 Hz, 1H, pyridin-5-H), 6.98(s, 1H, pyrazole-H), 5.89(br, 1H, NH), 4.28(m, 1H, CH), 2.27(s, 3H, thiophene-5-CH3), 2.23(s, 3H, thiophene-4-CH3), 1.28(d, J=6.9 Hz, 6H, 2CH3) |
| 1h | 10.43(s, 1H, NH), 8.49(dd, J=1.5, 4.8 Hz, 1H, pyridin-6-H), 7.91(dd, J=1.2, 4.8 Hz, 1H, pyridin-4-H), 7.43(dd, J=4.8, 8.4 Hz, 1H, pyridin-5-H), 6.96(s, 1H, pyrazole-H), 3.71(m, 4H, morpholine-2,6-2CH2), 3.54(m, 4H, morpholine-3,5-2CH2), 2.22(s, 3H, thiophene-5-CH3), 2.04(s, 3H, thiophene-4-CH3) |
| 2a | 8.33(s, 1H, pyrazole-H), 7.70(s, 1H, NH), 7.65(d, J=6.6 Hz, 2H, Ph-2,6-2H), 7.47(d, J=6.6 Hz, 2H, Ph-3,5-2H), 3.93(s, 3H, pyrazole-CH3), 2.93(s, 3H, CH3), 2.23(s, 3H, thiophene-5-CH3), 1.92(s, 3H, thiophene-4-CH3) |
| 2b | 8.23(s, 1H, pyrazole-H), 7.71(s, 1H, NH), 7.68(d, J=6.9 Hz, 2H, Ph-2,6-2H), 7.47(d, J=6.9 Hz, 2H, Ph-3,5-2H), 3.93(s, 3H, pyrazole-CH3), 2.83(s, 6H, 2CH3), 2.23(s, 3H, thiophene-5-CH3), 1.92(s, 3H, thiophene-4-CH3) |
| 2c | 12.01(s, 1H, NH), 8.31(s, 1H, pyrazole-H), 7.68(d, J=6.3 Hz, 2H, Ph-2,6-2H), 7.47(d, J=6.3 Hz, 2H, Ph-3,5-2H), 3.96(m, 1H, CH), 3.95(s, 3H, pyrazole-CH3), 2.23(s, 3H, thiophene-5-CH3), 2.15(s, 3H, thiophene-4-CH3), 1.13(s, 3H, CH3), 1.11(s, 3H, CH3) |
| 2d | 8.29(s, 1H, pyrazole-H), 7.71(s, 1H, NH), 7.66(d, J=6.6 Hz, 2H, Ph-2,6-2H), 7.43(d, J=6.6 Hz, 2H, Ph-3,5-2H), 3.93(s, 3H, pyrazole-CH3), 2.32(m, 1H, CH), 2.23(s, 3H, thiophene-5-CH3), 1.92(s, 3H, thiophene-4-CH3), 0.83(m, 4H, 2CH2) |
| 3a | 12.40(s, 1H, NH), 7.77—7.58(m, 4H, Ph), 6.05(s, 1H, NH), 2.95(d, J=3.0 Hz, 3H, CH3), 2.32(s, 6H, thiophene-2CH3) |
| 3b | 9.19(s, 1H, NH), 7.75—7.59(m, 4H, Ph), 3.02(s, 6H, 2CH3), 2.32(s, 3H, thiophene-5-CH3), 2.06(s, 3H, thiophene-4-CH3) |
| 3c | 12.31(s, 1H, NH), 7.76—7.58(m, 4H, Ph), 5.85(br, 1H, NH), 4.19(m, 1H, CH), 2.31(s, 6H, thiophene-2CH3), 1.24(d, J=5.1 Hz, 6H, 2CH3) |
| 3d | 12.39(s, 1H, NH), 7.77—7.58(m, 4H, Ph), 6.19(s, 1H, NH), 2.80(m, 1H, CH), 2.31(s, 3H, thiophene-5-CH3), 2.26(s, 3H, thiophene-4-CH3), 0.85(m, 4H, 2CH2) |
| 3e | 9.26(s, 1H, NH), 7.76—7.64(m, 4H, Ph), 3.78—3.46(m, 8H, morpholine), 2.31(s, 3H, thiophene-5-CH3), 2.07(s, 3H, thiophene-4-CH3) |
| Compd. | Mortality(%) | |
|---|---|---|
| 600 mg/L | 20 mg/L | |
| 1a | 100 | 70 |
| 1b | 100 | 40 |
| 1c | 100 | 50 |
| 1d | 100 | 30 |
| 1e | 100 | 60 |
| 1f | 100 | 50 |
| 1g | 100 | 50 |
| 1h | 100 | 20 |
| Chlomtraniliprole | 100 | 100 |
Table 3 Insecticidal activity against Plutella xylostella of the target compounds 1
| Compd. | Mortality(%) | |
|---|---|---|
| 600 mg/L | 20 mg/L | |
| 1a | 100 | 70 |
| 1b | 100 | 40 |
| 1c | 100 | 50 |
| 1d | 100 | 30 |
| 1e | 100 | 60 |
| 1f | 100 | 50 |
| 1g | 100 | 50 |
| 1h | 100 | 20 |
| Chlomtraniliprole | 100 | 100 |
| [1] | Liu C. L., Chin. J. Pestic., 2005, 44(11), 527 |
| (刘长令. 农药, 2005, 44(11), 527) | |
| [2] | Li Y., Li M., Chai B. S., Chin. J. Pestic., 2006, 45(10), 697—699 |
| (李洋, 李淼, 柴宝山. 农药, 2006, 45(10), 697—699) | |
| [3] | Dong W. L., Xu J. Y., Liu X. H., Zhao W. G., Li Z. M., Chin. J. Pest. Sci., 2008, 10(2), 178—185 |
| (董卫莉, 徐俊英, 刘幸海, 赵卫光, 李正名. 农药学学报, 2008, 10(2), 178—185) | |
| [4] | Liu J. B., Li Y. X., Chen Y. W., Wu C. C., Wan Y. Y., Wei W., Xiong L. X., Zhang X., Yu S. J., Li Z. M., Chem. Res. Chinese Universities,2016, 32(1), 41—48 |
| [5] | Zhang X. L., Liu A. L., Zhao Y., Xiong L. X., Li Z. M., Chem. Res. Chinese Universities,2013, 29(6), 1134—1139 |
| [6] | Zhang X. L., Wang B. L., Mao M. Z., Xiong L. X., Yu S. J., Li Z. M., Chem. J. Chinese Universities,2013, 34(1), 96—102 |
| (张秀兰, 王宝雷, 毛明珍, 熊丽霞, 于淑晶, 李正名. 高等学校化学学报,2013, 34(1), 96—102) | |
| [7] | Wu T. T., Pesticidal 5-Amino-4-ethylsulfinyl-1-arylpyrazoles, US 5814652A, 1998-09-29 |
| [8] | Guan A. Y., Liu C. L., Yang X. P., Dekeyser M., Chem. Rev., 2014, 114(14), 7079—7107 |
| [9] | Li M., Liu C. L., Zhang J., Wu Q., Hao S. L., Song Y. Q., Pest Manag. Sci., 2013, 69(5), 635—641 |
| [10] | Li M., Liu C. L., Li L., Yang H., Li Z. N., Zhang H., Li Z. M., Pest Manag. Sci., 2010, 66(1), 107—112 |
| [11] | LiuC. L., Guan A. Y., Yang J. D., Chai B. S., Li M., Li H. C., Yang J. C., Xie Y., J. Agric. Food Chem., 2016, 64(1), 45—51 |
| [12] | Chai B. S., Liu C. L., Li H. C., He X. M., Luo Y. M., Huang G., Zhang H., Chang J. B., Pest Manag. Sci., 2010, 66(11), 1208—1214 |
| [13] | Chai B. S., Liu C. L., Li H. C., Liu S. W., Huang G., Song Y. Q., Chang J. B., Pest Manag. Sci., 2011, 67(9), 1141—1146 |
| [14] | Li H. C., Guan A. Y., Huang G., Liu C. L., Li Z. N., Xie Y., Lan J., Bioorg. Med. Chem., 2016, 24(3), 453—461 |
| [15] | Yang J. C., Li M., Wu Q., Liu C. L., Chang X. H., Bioorg. Med. Chem., 2016, 24(3), 383—390 |
| [16] | Xie Y., Chi H. W., Guan A. Y., Liu C. L., Ma H. J., Cui D. L., Bioorg. Med. Chem., 2016, 24(3), 428—434 |
| [17] | Guan A. Y., Liu C. L., Sun X. F., Xie Y., Bioorg. Med. Chem., 2016, 24(3), 342—353 |
| [18] | Lahm G. P., Selby T. P., Freudenberger J. H., Stevenson T. M., Bioorg. Med. Chem. Lett., 2005, 15(21), 4898—4906 |
| [19] | Coppola G. M., J. Heterocyclic Chem., 1999, 36(3), 563—588 |
| [20] | Lahm G. P., Selby T. P., Stevenson T. M., Arthropodicidal Anthranilamides WO 03015519A1,2003-02-27 |
| [21] | LahmG. P., Novel Anthranilamides Useful for Controlling Invertebrate Pests, WO 2006023783A1, 2006-03-02 |
| [1] | 李然, 张旭东, 穆丽丹, 孙童, 艾刚刚, 沙夜龙, 张玉琦, 王记江. 三联噻吩衍生物功能化SiO2反蛋白石光子晶体荧光薄膜的制备及应用[J]. 高等学校化学学报, 2021, 42(9): 2989. |
| [2] | 张仁丽, 王瑶, 遇治权, 孙志超, 王安杰, 刘颖雅. 氟改性UiO-66固载钼基过氧化物催化氧化含硫化合物[J]. 高等学校化学学报, 2021, 42(6): 1914. |
| [3] | 李康明,陈佳,易阳杰,闫忠忠,叶姣,龙楚云,柳爱平,胡艾希,李建明. 1-(4-氯苯基)-2-环丙基酮肟醚的设计、 合成及杀虫活性[J]. 高等学校化学学报, 2020, 41(5): 1026. |
| [4] | 李康明, 李延赛, 易阳杰, 徐雷涛, 叶姣, 欧晓明, 李建明, 胡艾希. 5-吡唑甲酰胺类衍生物的设计、 合成与生物活性[J]. 高等学校化学学报, 2020, 41(4): 716. |
| [5] | 欧阳密, 陈璐, 胡旭明, 李裕文, 戴大程, 屠袁波, 白茹, 吕晓静, 张诚. TiO2-PTPAT纳米核/壳结构复合膜的制备及电致变色性能[J]. 高等学校化学学报, 2020, 41(12): 2796. |
| [6] | 王影, 孙传胤, 王润伟, 张震东, 张大明, 张宗弢, 裘式纶. 双亲型Ti/ZSM-5分子筛催化剂的制备及氧化脱硫性能[J]. 高等学校化学学报, 2019, 40(6): 1265. |
| [7] | 陈红, 杜勇慧, 张鑫, 刘文闫, 周晓明. 聚(3-己基噻吩)包覆富锂层状正极材料Li1.18Ni0.15Co0.15Mn0.52O2的制备与电化学性能[J]. 高等学校化学学报, 2019, 40(4): 777. |
| [8] | 钱明, 徐志民, 刘炜炜, 韩冰. 吡咯-(3,4-乙烯二氧噻吩)共聚物有序纳米阵列薄膜的制备及表征[J]. 高等学校化学学报, 2019, 40(3): 612. |
| [9] | 曲星星, 戴玉玉, 李维军, 欧阳密, 张诚. 噻吩-咔唑-苯衍生物的设计、 合成及电致变色性质[J]. 高等学校化学学报, 2018, 39(8): 1699. |
| [10] | 赵邦屯, 陶晶晶, 陈小纪, 付慧敏, 朱卫民. 含噻吩和吡啶基的插烯式四硫富瓦烯衍生物的合成、 结构和电化学性质[J]. 高等学校化学学报, 2018, 39(7): 1449. |
| [11] | 王凤燕, 晏妮, 魏俊基, 夏慧芸, 宋莉芳, 宋家乐, 高莉宁, 颜录科. 水溶性聚噻吩衍生物的合成、表征及细胞成像[J]. 高等学校化学学报, 2018, 39(11): 2594. |
| [12] | 张思航, 何永锋, 付润芳, 蒋洁, 李晴碧, 顾迎春, 陈胜. 纳米纤维素/聚3,4-乙撑二氧噻吩复合薄膜的制备及电致变色性能[J]. 高等学校化学学报, 2017, 38(6): 1090. |
| [13] | 吕耀康, 刘幼幼, 潘云, 刘刚, 陈钧, 郭芸, 初文静, 慎炼, 张诚. 基于3,4-乙烯二氧噻吩和吡咯-3-甲酸的共聚物薄膜的制备、表征及电致变色性能[J]. 高等学校化学学报, 2017, 38(3): 484. |
| [14] | 范建训, 冀利妃, 任爱民. 吡咯并吡咯二酮与低聚噻吩共聚物电荷传输性质的理论研究[J]. 高等学校化学学报, 2017, 38(11): 2053. |
| [15] | 孙太强, 李斌, 聂尧, 王栋, 徐岩. 羰基还原酶CR2重组酶体系不对称合成(S)-N,N-二甲基-3-羟基-3-(2-噻吩)-1-丙胺[J]. 高等学校化学学报, 2017, 38(10): 1772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||