

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (1): 227.doi: 10.7503/cjcu20200413
所属专题: 分子筛功能材料 2021年,42卷,第1期
郭淑佳1,2,王森1,张莉1,2,秦张峰1,王鹏飞1,董梅1,王建国1,2,樊卫斌1
收稿日期:2020-07-01
出版日期:2021-01-10
发布日期:2021-01-12
基金资助:
GUO Shujia1,2, WANG Sen1( ), ZHANG Li1,2, QIN Zhangfeng1(
), ZHANG Li1,2, QIN Zhangfeng1( ), WANG Pengfei1, DONG Mei1, WANG Jianguo1,2, FAN Weibin1(
), WANG Pengfei1, DONG Mei1, WANG Jianguo1,2, FAN Weibin1( )
)
Received:2020-07-01
Online:2021-01-10
Published:2021-01-12
Contact:
WANG Sen,QIN Zhangfeng,FAN Weibin
E-mail:wangsen@sxicc.ac.cn;qzhf@sxicc.ac.cn;fanwb@sxicc.ac.cn
Supported by:摘要:
ZSM-5分子筛在甲醇制烯烃(MTO)过程中的催化性能和反应机理与其孔道中酸位点分布位置紧密相关. 本文证明在水热合成过程中加入适量的钠离子(Na+)可以增加ZSM-5分子筛交叉腔酸位点比例; 从而促进高级甲基苯的生成并加速芳烃循环, 有利于乙烯生成. 相反, 在合成过程中不添加钠离子, 所制备的ZSM-5分子筛直孔道和正弦孔道酸位点比例明显提高, 有利于促进烯烃循环并提高丙烯和C3+烯烃选择性.
中图分类号:
TrendMD:
郭淑佳, 王森, 张莉, 秦张峰, 王鹏飞, 董梅, 王建国, 樊卫斌. ZSM-5分子筛酸位分布及其甲醇制烯烃催化性能的定向管理与调控. 高等学校化学学报, 2021, 42(1): 227.
GUO Shujia, WANG Sen, ZHANG Li, QIN Zhangfeng, WANG Pengfei, DONG Mei, WANG Jianguo, FAN Weibin. Regulating the Acid Sites Distribution in ZSM-5 Zeolite and Its Catalytic Performance in the Conversion of Methanol to Olefins. Chem. J. Chinese Universities, 2021, 42(1): 227.
| Zeolite | Crystallinity (%) | n(Si)/n(Al) | Surface area/(m2·g-1) | Pore volume/(cm3·g-1) | ||
|---|---|---|---|---|---|---|
| Total | Micro | Total | Micro | |||
| ZSM-5-0Na | 100 | 36.1 | 430.2 | 294.1 | 0.37 | 0.11 |
| ZSM-5-0.2Na | 93.5 | 35.0 | 364.6 | 275.6 | 0.24 | 0.12 |
| ZSM-5-0.4Na | 92.9 | 35.6 | 399.5 | 312.6 | 0.25 | 0.10 |
| ZSM-5-0.6Na | 92.4 | 33.5 | 351.4 | 277.0 | 0.22 | 0.08 |
Table 1 Chemical composition and textural properties of all ZSM-5 zeolites*
| Zeolite | Crystallinity (%) | n(Si)/n(Al) | Surface area/(m2·g-1) | Pore volume/(cm3·g-1) | ||
|---|---|---|---|---|---|---|
| Total | Micro | Total | Micro | |||
| ZSM-5-0Na | 100 | 36.1 | 430.2 | 294.1 | 0.37 | 0.11 |
| ZSM-5-0.2Na | 93.5 | 35.0 | 364.6 | 275.6 | 0.24 | 0.12 |
| ZSM-5-0.4Na | 92.9 | 35.6 | 399.5 | 312.6 | 0.25 | 0.10 |
| ZSM-5-0.6Na | 92.4 | 33.5 | 351.4 | 277.0 | 0.22 | 0.08 |
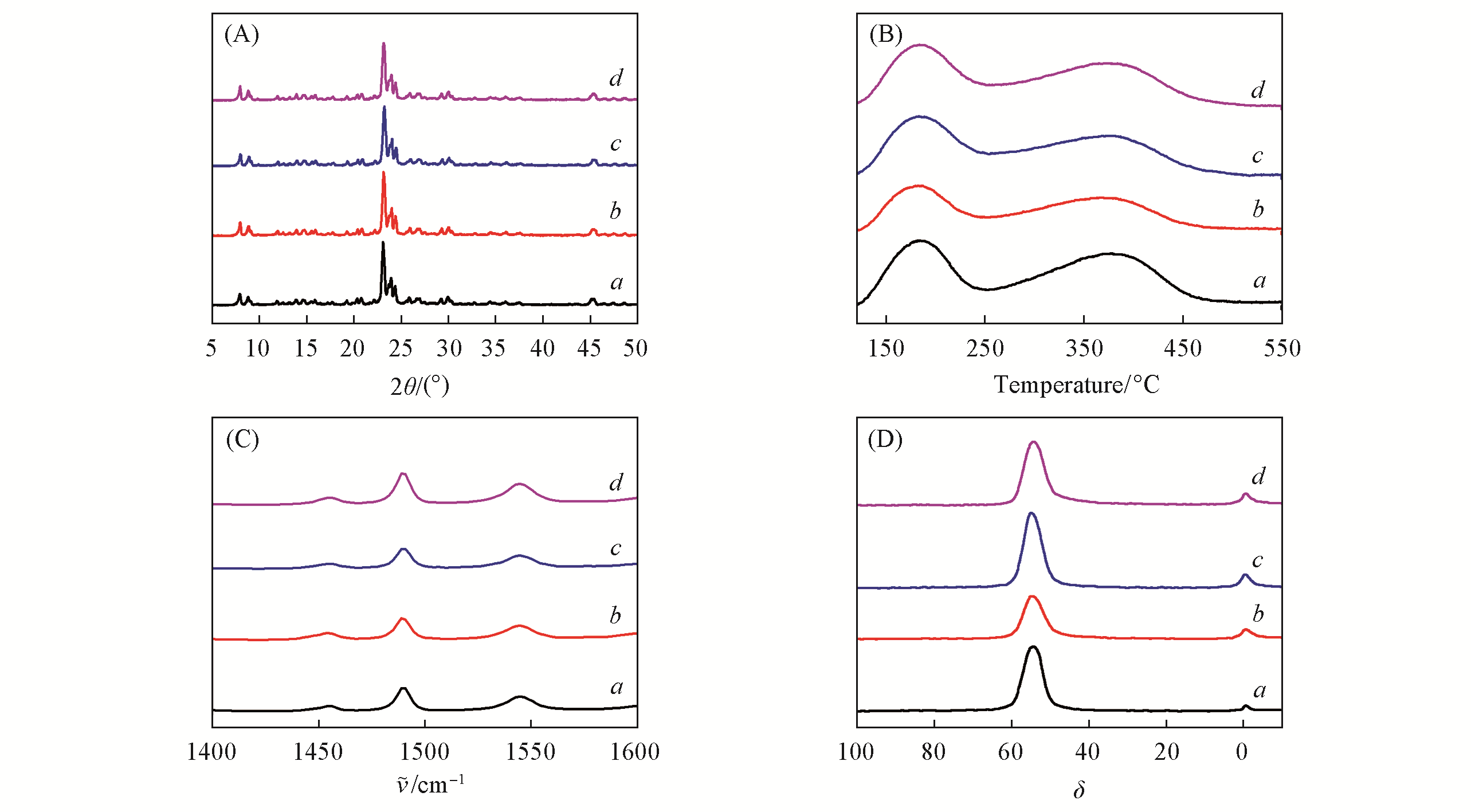
Fig.1 XRD patterns(A), NH3?TPD profiles(B), Py?IR spectra collected at 423 K(C) and 27Al solid?state MAS NMR spectra(D) of ZSM?5?0Na(a), ZSM?5?0.2Na(b), ZSM?5?0.4Na(c) and ZSM?5?0.6Na(d)
| Zeolite | Acidity(423 K)/(μmol·g-1) | Acidity(523 K)/(μmol·g-1) | Acidity(623 K)/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | |
| ZSM?5?0Na | 220.0 | 178.4 | 41.6 | 186.0 | 158.5 | 27.5 | 154.5 | 132.3 | 22.2 |
| ZSM?5?0.2Na | 206.4 | 150.9 | 55.5 | 158.5 | 131.3 | 27.2 | 129.0 | 105.6 | 23.4 |
| ZSM?5?0.4Na | 204.0 | 162.6 | 41.4 | 160.5 | 135.9 | 24.6 | 132.7 | 113.1 | 19.6 |
| ZSM?5?0.6Na | 241.1 | 200.3 | 40.8 | 201.3 | 169.9 | 31.4 | 158.9 | 133.9 | 25.0 |
Table 2 Acid property of different ZSM-5 zeolites determined by Py-IR*
| Zeolite | Acidity(423 K)/(μmol·g-1) | Acidity(523 K)/(μmol·g-1) | Acidity(623 K)/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | |
| ZSM?5?0Na | 220.0 | 178.4 | 41.6 | 186.0 | 158.5 | 27.5 | 154.5 | 132.3 | 22.2 |
| ZSM?5?0.2Na | 206.4 | 150.9 | 55.5 | 158.5 | 131.3 | 27.2 | 129.0 | 105.6 | 23.4 |
| ZSM?5?0.4Na | 204.0 | 162.6 | 41.4 | 160.5 | 135.9 | 24.6 | 132.7 | 113.1 | 19.6 |
| ZSM?5?0.6Na | 241.1 | 200.3 | 40.8 | 201.3 | 169.9 | 31.4 | 158.9 | 133.9 | 25.0 |
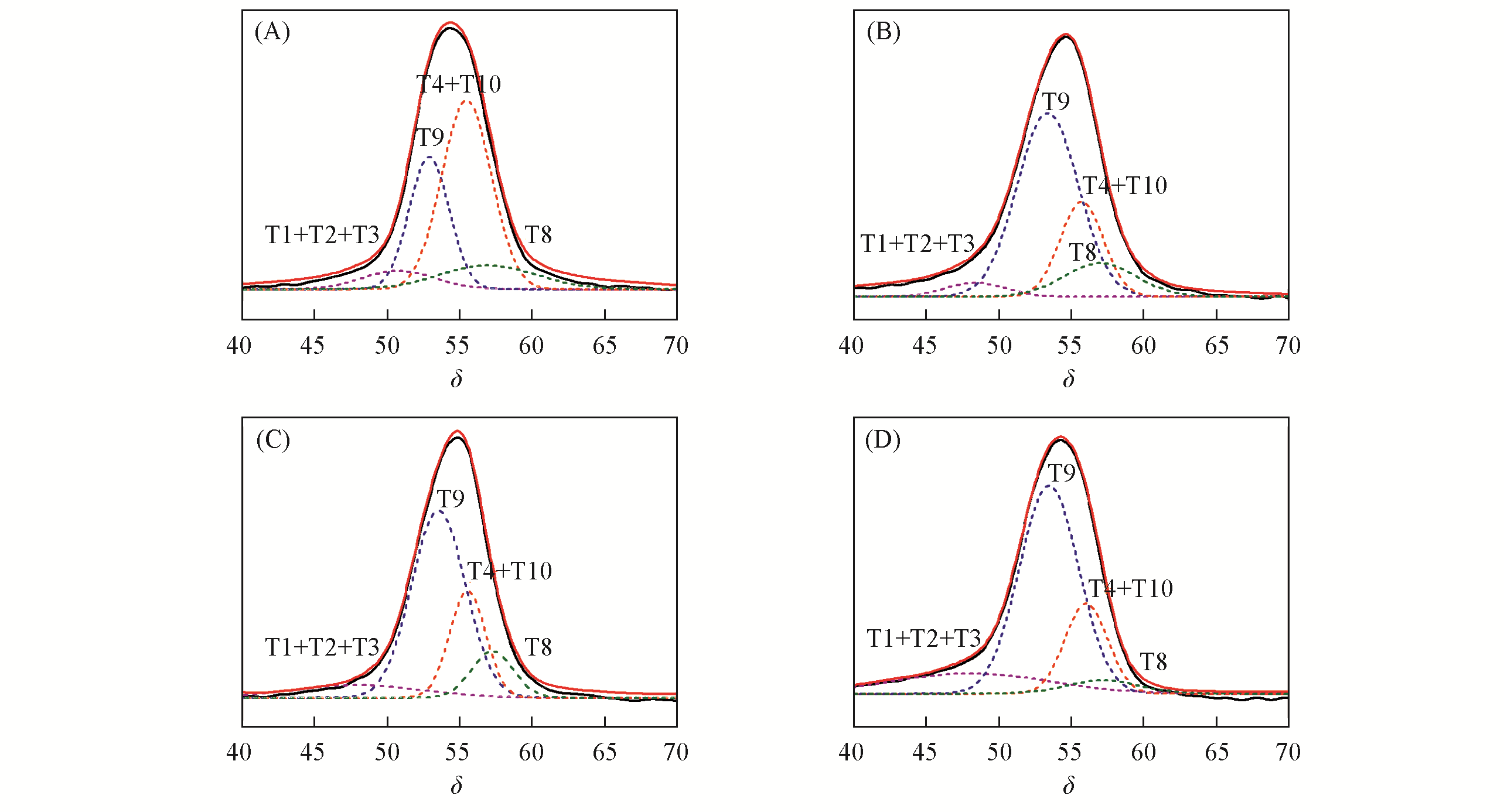
Fig.2 Deconvolution of the 27Al MAS NMR spectra of ZSM?5?0Na(A), ZSM?5?0.2Na(B), ZSM?5?0.4Na(C) and ZSM?5?0.6Na(D)The experimental spectra are shown in black lines and the fitted ones in red lines. The Al atoms at different T sites were assigned by referring the chemical shifts estimated by the DFT computation[11].
| Zeolite | AlEF(%) | AlF(%) | Al distribution(%) | |||
|---|---|---|---|---|---|---|
| δ 50.0 | δ 53.2 | δ 56.5 | δ 57.9 | |||
| ZSM?5?0Na | 2.4 | 97.6 | 7.6 | 28.7 | 50.7 | 13.0 |
| ZSM?5?0.2Na | 9.0 | 91.0 | 4.4 | 60.6 | 22.1 | 12.9 |
| ZSM?5?0.4Na | 8.2 | 91.8 | 9.4 | 57.5 | 22.0 | 11.1 |
| ZSM?5?0.6Na | 5.7 | 94.3 | 16.0 | 60.2 | 18.8 | 5.0 |
Table 3 Proportion of integrated peak area obtained by the deconvolution of 27Al MAS NMR spectra of ZSM-5 zeolites*
| Zeolite | AlEF(%) | AlF(%) | Al distribution(%) | |||
|---|---|---|---|---|---|---|
| δ 50.0 | δ 53.2 | δ 56.5 | δ 57.9 | |||
| ZSM?5?0Na | 2.4 | 97.6 | 7.6 | 28.7 | 50.7 | 13.0 |
| ZSM?5?0.2Na | 9.0 | 91.0 | 4.4 | 60.6 | 22.1 | 12.9 |
| ZSM?5?0.4Na | 8.2 | 91.8 | 9.4 | 57.5 | 22.0 | 11.1 |
| ZSM?5?0.6Na | 5.7 | 94.3 | 16.0 | 60.2 | 18.8 | 5.0 |
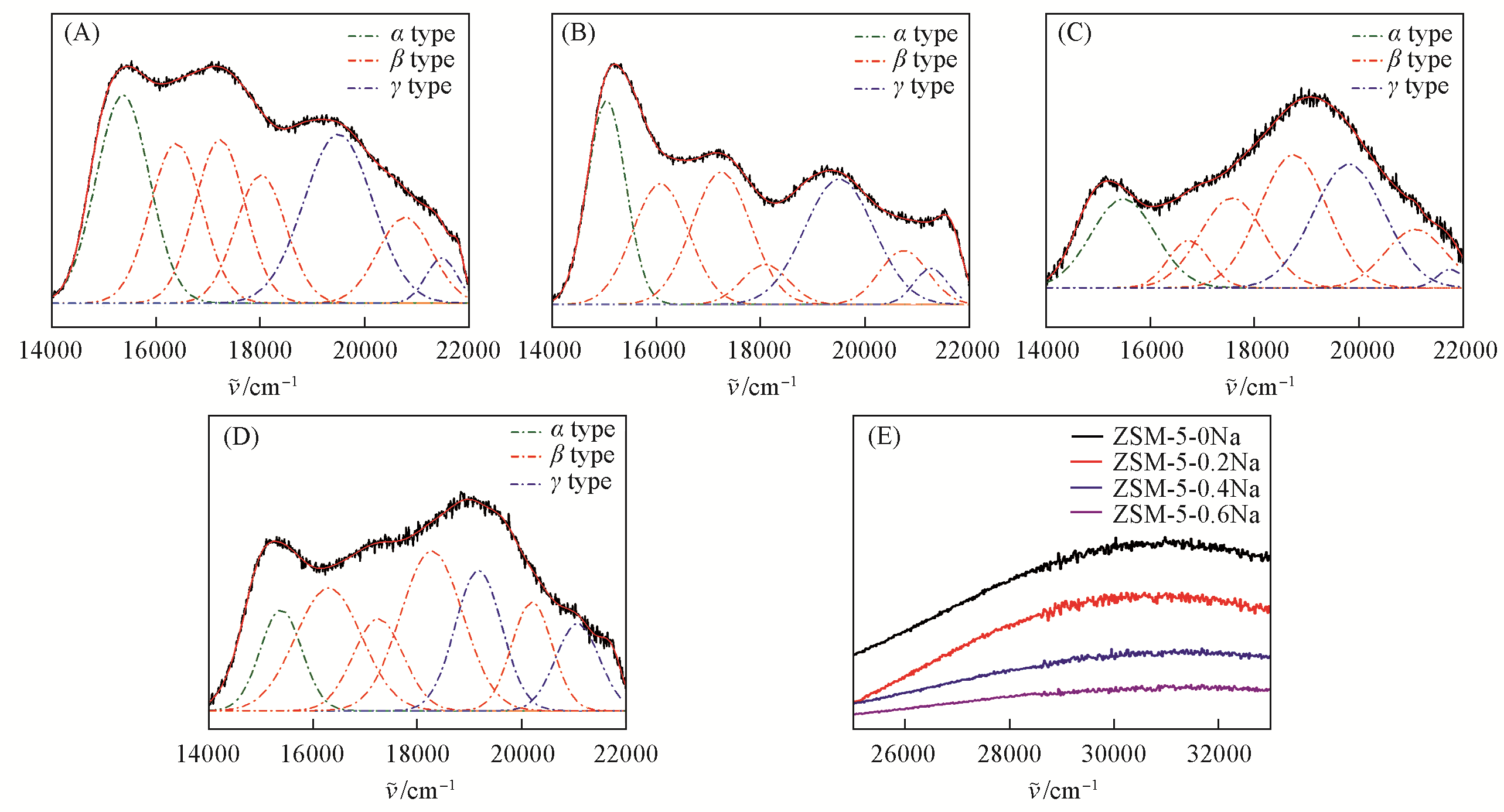
Fig.3 Deconvolution of the DR UV?Vis spectra of ZSM?5?0Na(A), ZSM?5?0.2Na(B), ZSM?5?0.4Na(C) and ZSM?5?0.6Na(D) and the region of 25000—33000 cm-1 of various Co2+?exchanged ZSM?5 zeolites(E)The experimental spectra are shown in black lines and the fitted ones in red lines.
| Zeolite | n(Si)/n(Al) | Alpairs(%) | Alsingle(%) | Al distribution(%) | ||
|---|---|---|---|---|---|---|
| α type | β type | γ type | ||||
| ZSM?5?0Na | 37.2 | 75.4 | 24.6 | 26.4 | 43.3 | 30.3 |
| ZSM?5?0.2Na | 35.9 | 83.1 | 16.9 | 21.1 | 58.6 | 20.3 |
| ZSM?5?0.4Na | 35.5 | 72.2 | 37.8 | 16.3 | 62.3 | 21.4 |
| ZSM?5?0.6Na | 37.1 | 80.0 | 20.0 | 14.0 | 77.0 | 9.0 |
Table 4 Distributions of different Al species in the ZSM-5 zeolite as measured by DR UV-Vis spectra of Co2+-exchanged ZSM-5 zeolites*
| Zeolite | n(Si)/n(Al) | Alpairs(%) | Alsingle(%) | Al distribution(%) | ||
|---|---|---|---|---|---|---|
| α type | β type | γ type | ||||
| ZSM?5?0Na | 37.2 | 75.4 | 24.6 | 26.4 | 43.3 | 30.3 |
| ZSM?5?0.2Na | 35.9 | 83.1 | 16.9 | 21.1 | 58.6 | 20.3 |
| ZSM?5?0.4Na | 35.5 | 72.2 | 37.8 | 16.3 | 62.3 | 21.4 |
| ZSM?5?0.6Na | 37.1 | 80.0 | 20.0 | 14.0 | 77.0 | 9.0 |
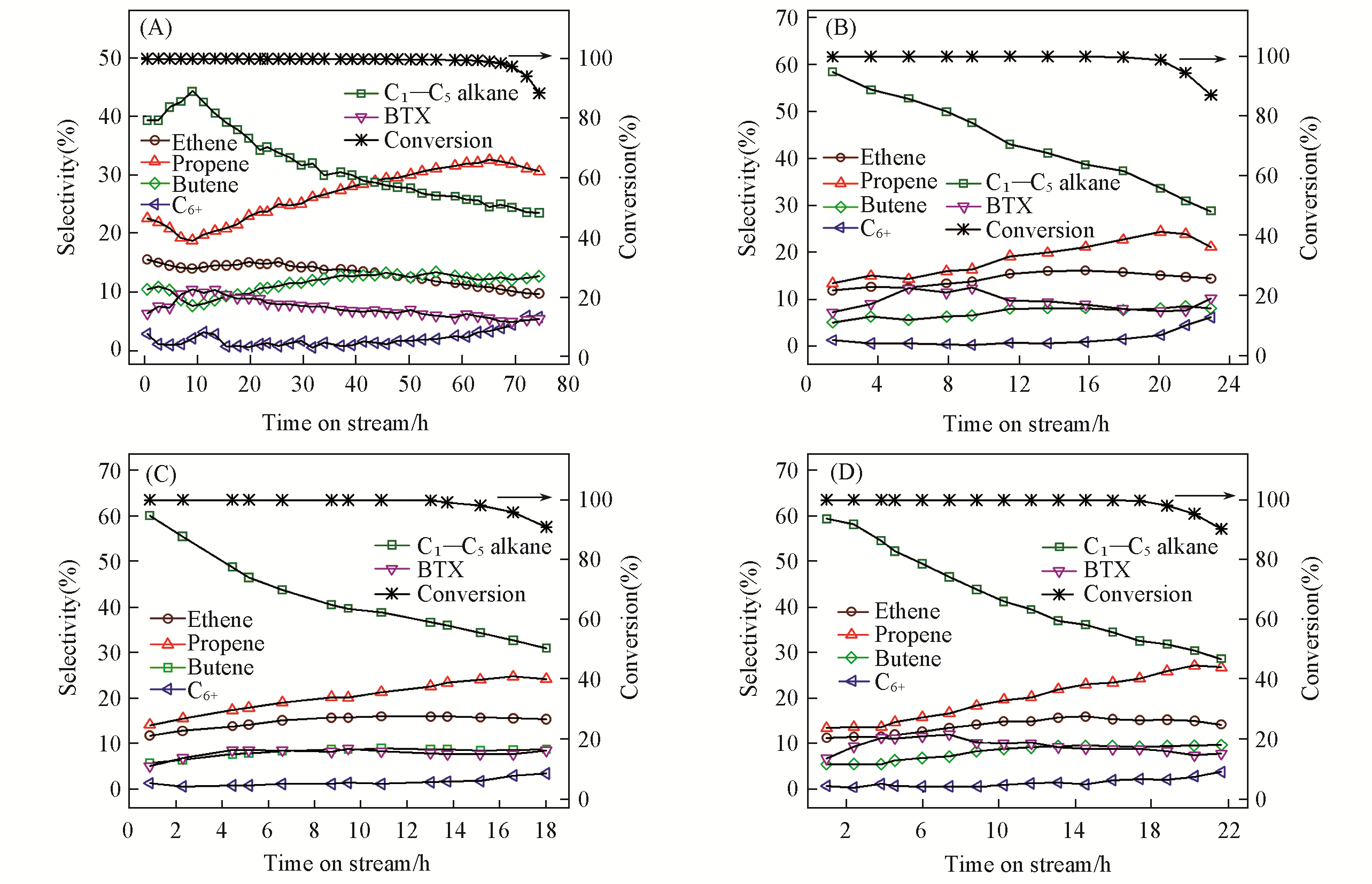
Fig.4 Variation of the methanol conversion and product selectivity with time on stream for MTO over the ZSM?5?0Na(A), ZSM?5?0.2Na(B), ZSM?5?0.4Na(C) and ZSM?5?0.6Na(D) zeolites under atmospheric pressure and 723 K, with a methanol WHSV of 3.8 h-1
| Zeolite | Conv. (%) | Product selectivity(%) | HTI | Lifetime /h | 10?4TON | (P-E)/E | 2MB/E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C | C | C1—C 5 | BTX | C4-HTI | C5-HTI | ||||||
| ZSM?5?0Na | 99.8 | 13.8 | 29.1 | 20.0 | 30.3 | 6.8 | 0.48 | 0.55 | 74.4 | 4.92 | 1.11 | 1.00 |
| ZSM?5?0.2Na | 99.9 | 15.5 | 19.2 | 12.6 | 43.0 | 9.7 | 0.69 | 0.67 | 22.9 | 1.77 | 0.23 | 0.67 |
| ZSM?5?0.4Na | 99.9 | 15.6 | 20.0 | 15.1 | 39.6 | 8.8 | 0.66 | 0.63 | 18.0 | 1.30 | 0.28 | 0.87 |
| ZSM?5?0.6Na | 99.9 | 14.8 | 19.6 | 14.2 | 41.5 | 9.9 | 0.67 | 0.63 | 21.6 | 1.27 | 0.32 | 0.82 |
Table 5 Catalytic test results for MTO over the ZSM-5 zeolites with different acid sites distributions*
| Zeolite | Conv. (%) | Product selectivity(%) | HTI | Lifetime /h | 10?4TON | (P-E)/E | 2MB/E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C | C | C1—C 5 | BTX | C4-HTI | C5-HTI | ||||||
| ZSM?5?0Na | 99.8 | 13.8 | 29.1 | 20.0 | 30.3 | 6.8 | 0.48 | 0.55 | 74.4 | 4.92 | 1.11 | 1.00 |
| ZSM?5?0.2Na | 99.9 | 15.5 | 19.2 | 12.6 | 43.0 | 9.7 | 0.69 | 0.67 | 22.9 | 1.77 | 0.23 | 0.67 |
| ZSM?5?0.4Na | 99.9 | 15.6 | 20.0 | 15.1 | 39.6 | 8.8 | 0.66 | 0.63 | 18.0 | 1.30 | 0.28 | 0.87 |
| ZSM?5?0.6Na | 99.9 | 14.8 | 19.6 | 14.2 | 41.5 | 9.9 | 0.67 | 0.63 | 21.6 | 1.27 | 0.32 | 0.82 |
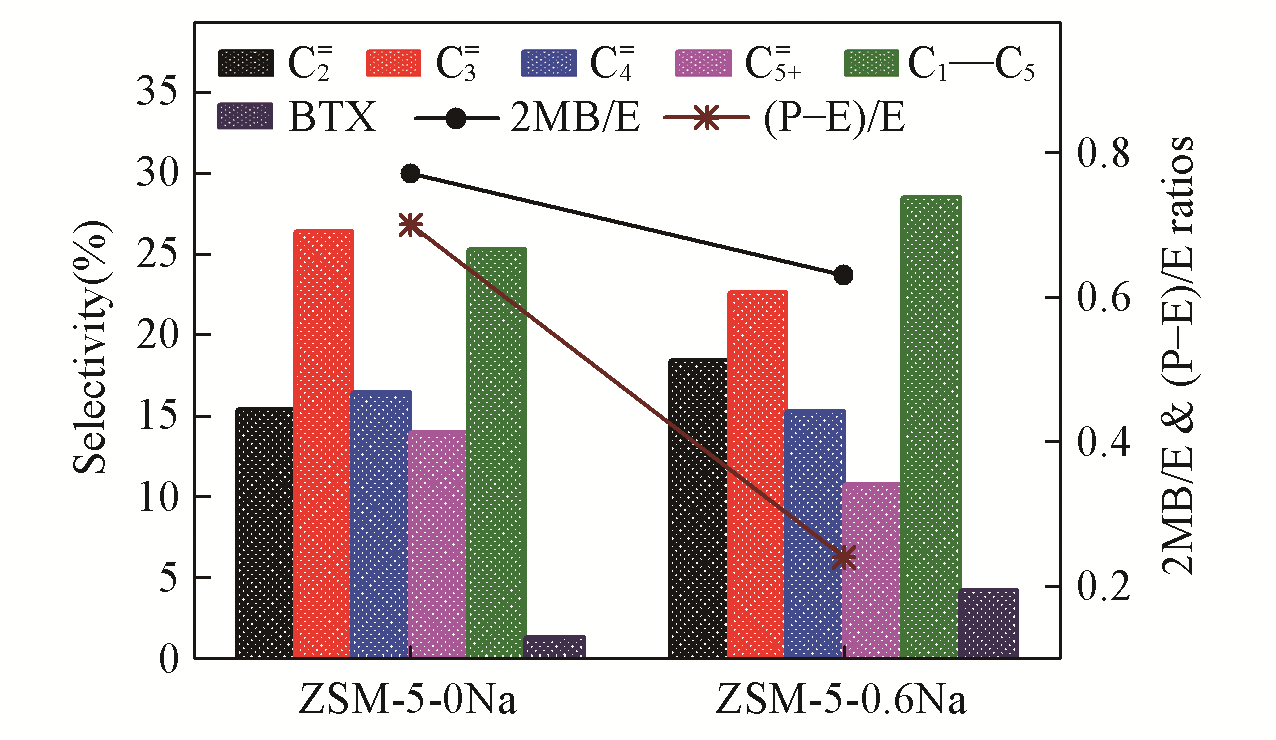
Fig.5 A comparison in the selectivities to ethene(C2???=), propene(C3???=), butene(C4???=), alkenes higher than butene(C5+=), C1―C5 alkanes, and benzene, toluene and xylenes(BTX) as well as the (P-E)/E and 2MB/E(P for propene, E for ethene, and 2MB for 2?methylbutane and 2?methyl?2?butene) for MTO over the ZSM?5?0Na and ZSM?5?0.6Na under atmospheric pressure and 623 K, with a WHSV of 48 h-1 and a methanol conversion of 75%, reported at 30 min on stream
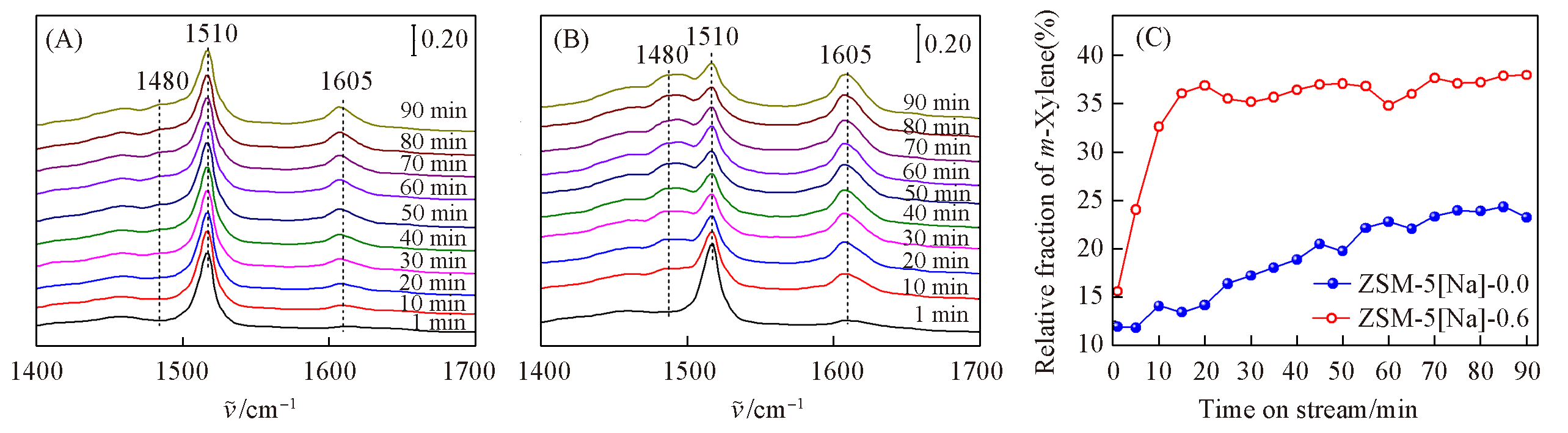
Fig.6 Time?resolved IR spectra of p?xylene isomerization over ZSM?5?0Na(A) and ZSM?5?0.6Na(B) at 473 K as well as the relative fraction of m?xylene(C)(C) Obtained with the peak area of m?xylene divided by the total peak areas of p?, o?, and m?xylenes in situ IR spectra. p?Xylene with a partial pressure of 300 Pa was continuously introduced into the reaction cell and the pressure was kept constant during the whole reaction process.
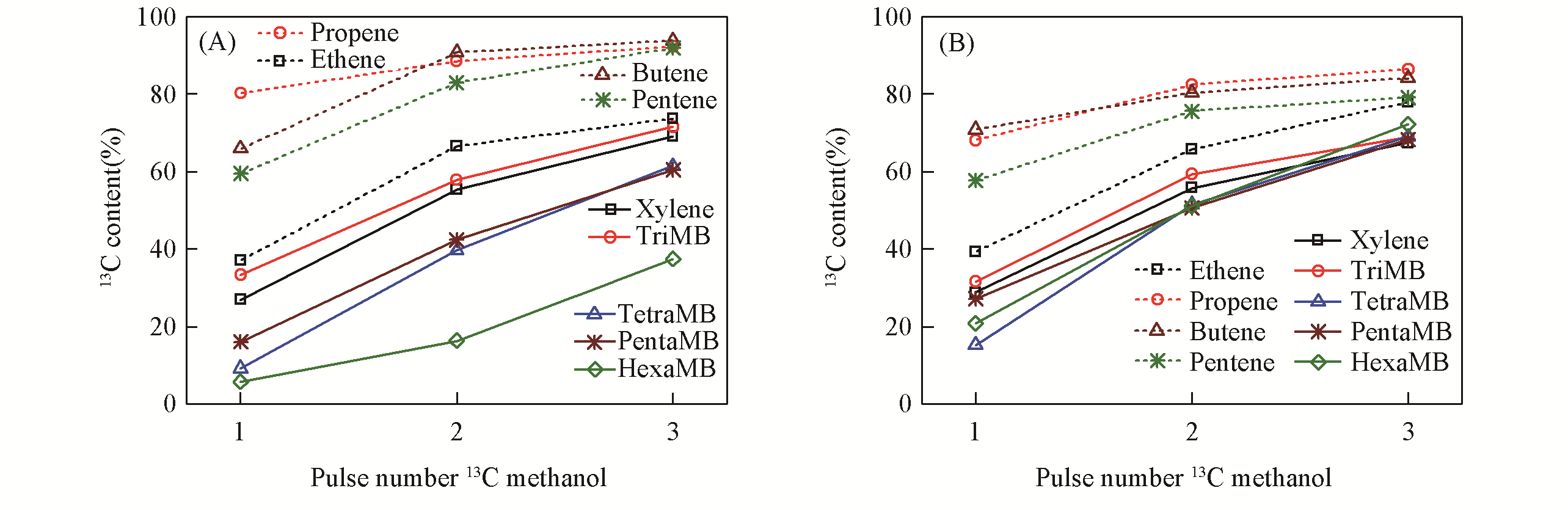
Fig.9 13C contents in the effluent olefins(ethene to pentene) and in the retained aromatics(polyMBs) for MTO over ZSM?5?0Na(A) and ZSM?5?0.6Na(B) zeolites at 553 K
| 1 | Haw J., Song W., Marcus D., Nicholas J., Acc. Chem. Res., 2003, 36, 317—326 |
| 2 | Olsbye U., Svelle S., Bjørgen M., Beato P., Janssens T. V. W., Joensen F., Bordiga S., Lillerud K. P., Angew. Chem. Int. Ed., 2012, 51(24), 5810—5831 |
| 3 | Tian P., Wei Y., Ye M., Liu Z., ACS Catal., 2015, 5(3), 1922—1938 |
| 4 | Xu S., Zhi Y., Han J., Zhang W., Wu X., Sun T., Wei Y., Liu Z., Adv Catal., 2017, 61, 37—122 |
| 5 | Wang C., Chu Y., Zheng A., Xu J., Wang Q., Gao P., Qi G., Gong Y., Deng F., Chem. Eur. J., 2014, 20(39), 1—13 |
| 6 | Dai W., Wan C., Dyballa M., Wu G., Guan N., Li L., Xie Z., Hunger M., ACS Catal., 2015, 5(1), 317—326 |
| 7 | Dedecek J., Sobalik Z., Wichterlova B., Catal. Rev. Sci. Eng., 2012, 54, 135—223 |
| 8 | Knott B., Nimlos C., Robichaud D., Nimlos M., Kim S., Gounder R., ACS Catal., 2018, 8(2),770—784 |
| 9 | Wang S., He Y., Jiao W., Wang J., Fan W., Curr. Opin. Chem. Eng., 2019,23, 146—154 |
| 10 | Chen J., Liang T., Li J., Wang S., Qin Z., Wang P., Huang L., Fan W., Wang J., ACS Catal., 2016, 6(4), 2299—2313 |
| 11 | Wang S., Wang P., Qin Z., Chen Y., Dong M., Li J., Zhang K., Liu P., Wang J., Fan W., ACS Catal., 2018, 8(6), 5485—5505 |
| 12 | Liang T., Chen J., Qin Z., Li J., Wang P., Wang S., Wang G., Dong M., Fan W., Wang J., ACS Catal., 2016, 6(11), 7311—7325 |
| 13 | Dedecek J., Balgova V., Pashkova V., Klein P., Wichterlova B., Chem. Mater., 2012, 24(16), 3231—3239 |
| 14 | Yokoi T., Mochizuki H., Namba S., Kondo J. N., Tatsumi T., J. Phys. Chem. C, 2015, 119(27), 15303—15315 |
| 15 | Pashkovava V., Sklenak S., Klein P., Urbanova M., Dedecek J., Chem. Eur. J., 2016, 22(12), 3937 —3941 |
| 16 | Di Iorio J., Gounder R., Chem. Mater., 2016, 28(7),2236—2247 |
| 17 | Zhao X., Wang L., Li J., Xu S., Zhang W., Wei Y., Guo X., Tian P., Liu Z., Catal. Sci. Technol., 2017, 7(4), 5882—5892 |
| 18 | Sazama P., Dedecek J., Gabova V., Wichterlova B., Spoto G., Bordiga S., J. Catal., 2008, 254(2), 180—189 |
| 19 | Wang S., Zhang L., Li S., Qin Z., Shi D., He S., Yuan K., Wang P., Zhao T., Fan S., Dong M., Li J., Fan W., J. Catal., 2019, 377, 81—97 |
| 20 | Price G., Iglesia E., Ind. Eng. Chem. Res., 1989, 28(6), 839—844 |
| 21 | Vjunov A., Fulton J. L., Huthwelker T., Pin S., Mei D. H., Schenter G. K., Govind N., Camaioni D. M., Hu J. Z., Lercher J. A., J. Am. Chem. Soc., 2014, 136(23), 8296-8306 |
| 22 | Liu Z., Dong X., Zhu Y., Emwas A., Zhang D., Tian Q., Han Y., ACS Catal., 2015, 5(10), 5837—5845 |
| 23 | Dedecek J., Lucero M. J., Li C. B., Gao F., Klein P., Urbanova M., Tvaruzkova Z., Sazama P., Sklenak S., J. Phys. Chem. C, 2011, 115(22), 11056—11064 |
| 24 | Sklenak S., Dedecek J., Li C. B., Wichterlova B., Gabova V., Sierka M., Sauer J., Angew. Chem. Int. Ed., 2007, 46(38), 7286—7289 |
| 25 | Zhao R., Zhao Z., Li S., Zhang W., J. Phys. Chem. Lett., 2017, 8(10), 2323—2327 |
| 26 | Pashkovava V., Sklenak S., Klein P., Urbanova M., Dedecek J., Chem. Eur. J., 2016, 22(12), 3937—3941 |
| 27 | Ilias S., Khare R., Malek A., Bhan A., J. Catal., 2013, 303, 135—140 |
| 28 | Bleken F., Janssens T. V. W., Svelle S., Olsbye U., Microporous Mesoporous Mater., 2012, 164, 190—198 |
| 29 | Shen Y., Le T., Fu D., Schmidt J., Filez M., Weckhuysen B., Rimer J., ACS Catal., 2018, 8(12), 11042—11053 |
| 30 | Gomez⁃Hortiguela L., Pinar A., Cora F., Perea⁃Pariente J., Chem. Commun., 2010, 46(12), 2073—2075 |
| 31 | Pinar A., Marquez⁃Alvarez C., Grande⁃Casas M., Perez⁃Pariente J., J. Catal., 2009, 263(2), 258—265 |
| 32 | Zheng S., Jentys A., Lercher J. A., J. Catal., 2006, 241(2), 304—311 |
| 33 | Bjørgen M., Bonino F., Kolboe S., Lillerud K., Zecchina A. Bordiga S., J. Am. Chem. Soc., 2003, 125(51), 15863—15868 |
| 34 | Dai W., Wu G., Li L., Guan N., Hunger M., ACS Catal., 2013, 3(4), 588—596 |
| 35 | Borodina E., Meirer F., Lezcano⁃Gonzalez I., Mokhtar M., Asiri A., Al⁃Thabaiti S., Basahel S., Ruiz⁃Martinez J., Weckhuysen B., ACS Catal., 2015, 5(2), 992—1003 |
| 36 | Goetze J., Meirer F., Yarulina I., Gascon J., Kapteijn F., Ruiz⁃Martinez J., Weckhuysen B., ACS Catal., 2017, 7(6), 4033—4046 |
| 37 | Van Speybroeck V., Hemelsoet K., De Wispelaere K., Qian Q., Van der Mynsbrugge J., De Sterck B., Weckhuysen B., Waroquier M., ChemCatChem, 2013, 5(1), 173—184 |
| 38 | Wang S., Chen Y., Qin Z., Zhao T., Fan S., Dong M., Li J., Fan W., Wang J., J. Catal., 2019, 369, 382—395 |
| 39 | Xu T., Barich D., Goguen P., Song W., Wang Z., Nicholas J., Haw J., J. Am. Chem. Soc., 1998, 120(16), 4025—4026 |
| 40 | Haw J., Nicholas J., Song W., Deng F., Wang Z., Xu T., Heneghan C., J. Am. Chem. Soc., 2000, 122(19), 4763—4775 |
| 41 | Li J., Wei Y., Chen J., Tian P., Su X., Xu S., Qi Y., Wang Q., Zhou Y., He Y., Liu Z., J. Am. Chem. Soc., 2012, 134(2), 836— 839 |
| 42 | Wang C., Xu J., Qi G., Gong Y., Wang W., Gao P., Wang Q., Feng N., Liu X., Deng F., J. Catal., 2015, 332, 127—137 |
| 43 | Zhang W., Chen J., Xu S., Chu Y., Wei Y., Zhi Y., Huang J., Zhang A., Wu X., Meng X., Xiao F., Deng F., Liu Z., ACS Catal., 2018, 8(12), 10950—10963 |
| [1] | 於霞, 宋晨海, 郭向可, 薛念华, 丁维平. HZSM-5分子筛中邻近酸中心上的协同催化作用[J]. 高等学校化学学报, 2021, 42(1): 239. |
| [2] | 边凯, 候章贵, 段欣瑞, 李孝国, 常洋, 曹辉, 张安峰, 郭新闻. 二维HZSM-5纳米片的合成及催化苯与稀乙烯烷基化制乙苯[J]. 高等学校化学学报, 2019, 40(4): 784. |
| [3] | 孙思齐,王影,孙传胤,王润伟,张震东,张宗弢,裘式纶. 碗状双亲型ZSM-5分子筛负载金纳米粒子的制备及催化性能[J]. 高等学校化学学报, 2019, 40(12): 2436. |
| [4] | 张瑞珍, 王翠, 邢普, 温少波, 王剑, 赵亮富, 李玉平. 多级孔HZSM-5分子筛催化剂的制备及低碳烃芳构化应用[J]. 高等学校化学学报, 2015, 36(4): 725. |
| [5] | 张前, 陈春影, 丁双, 张文婷, 许迪欧, 唐晓建, 王润伟, 姜日花, 张宗弢, 裘式纶. 一步水热合成Mn-ZSM-5纳米分子筛及其性能[J]. 高等学校化学学报, 2012, 33(03): 453. |
| [6] | 张强, 李春义, 山红红, 杨朝合. 预先负载原料法合成ZSM-5/SAPO-5复合分子筛[J]. 高等学校化学学报, 2011, 32(12): 2721. |
| [7] | 靳凤英 郭新闻 王祥生. 乙二胺四乙酸改性对NiMo/Nano-HZSM-5催化剂脱硫性能的影响[J]. 高等学校化学学报, 2011, 32(1): 113. |
| [8] | 童伟益, 刘志成, 孔德金, 祁晓岚, 郭杨龙. 核壳型复合分子筛ZSM-5/Nano-β的合成与表征[J]. 高等学校化学学报, 2009, 30(5): 959. |
| [9] | 武光军, 王鑫, 于爱敏, 王贵昌, 杨雅莉, 章福祥, 关乃佳. 含氮ZSM-5分子筛骨架中氮取代位置的密度泛函计算[J]. 高等学校化学学报, 2008, 29(12): 2403. |
| [10] | 颜桂炀, 王绪绪, 付贤智. ZSM-5分子筛光催化活性的初步研究[J]. 高等学校化学学报, 2004, 25(5): 942. |
| [11] | 贾明君, 张文祥, 肖丰收, 吴通好, 孙铁. 分散法制备的CuCl/ZSM-5分子筛催化剂上C3H6选择性催化还原NO反应的研究[J]. 高等学校化学学报, 1998, 19(4): 606. |
| [12] | 马淑杰, 李连生, 孙富平, 裘式伦. 双杂原子Ti-Fe-ZSM-5分子筛的合成与表征[J]. 高等学校化学学报, 1997, 18(4): 504. |
| [13] | 常春, 张武阳, 张丽华, 李荣生, 田明文, 杨胥微, 侯莉. 微波辐射下稀土交换ZSM-5分子筛的制备及裂解反应研究[J]. 高等学校化学学报, 1996, 17(12): 1914. |
| [14] | 陶克毅, 高峰, 温朗友, 陈忠明, 臧雅茹, 李赫咺. (K,Na)ZSM-5的酸碱性质及催化性能[J]. 高等学校化学学报, 1992, 13(10): 1274. |
| [15] | 庞文琴, 于龙, 吴亚平. 微酸性氟离子体系Zr-ZSM-5型分子筛合成与结构研究[J]. 高等学校化学学报, 1989, 10(9): 951. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||