

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (6): 1277.doi: 10.7503/cjcu20200086
收稿日期:2020-02-19
出版日期:2020-06-10
发布日期:2020-03-27
通讯作者:
郭景富,任爱民
E-mail:guojf217@nenu.edu.cn;aimin_ren@yahoo.com
基金资助:
LIN Guifeng1,GUO Jingfu1,*( ),HE Tengfei2,REN Aimin2,*(
),HE Tengfei2,REN Aimin2,*( )
)
Received:2020-02-19
Online:2020-06-10
Published:2020-03-27
Contact:
Jingfu GUO,Aimin REN
E-mail:guojf217@nenu.edu.cn;aimin_ren@yahoo.com
Supported by:摘要:
设计了一系列具有不同供电子基团的N-苯基-1,8-萘二甲酰亚胺衍生物(NNI-R), 对它们在二氯甲烷和气相中的几何结构、 电子结构以及室温磷光性能进行了研究. 在二氯甲烷极性溶剂中, NNI-R系列分子的最低单重激发态(S1)有2个异构体, 分别表现为局域激发(LE)和电荷转移激发(CT). 具有弱给电子体(R=OMe, OH)时的NNI-R分子, 其S1态为LE结构, 给体和受体间二面角垂直, 其总能量远低于CT结构, 会抑制系间窜越(ISC)的发生, 不会发生磷光现象. 在气相下, NNI-R系列分子的S1态只有一种稳定的CT结构, 该特征能显著抑制荧光发射, 并有效促进系间窜越, 使NNI-R系列分子的室温磷光发射成为一种可能.
中图分类号:
TrendMD:
林桂锋,郭景富,何腾飞,任爱民. 供电子基团修饰对NNI-R系列分子光物理性质的影响. 高等学校化学学报, 2020, 41(6): 1277.
LIN Guifeng,GUO Jingfu,HE Tengfei,REN Aimin. Influences of Electron Donating Groups on the Photophysical Properties of NNI-R Series Molecules †. Chem. J. Chinese Universities, 2020, 41(6): 1277.
| Molecule | Dihedral anglea/(°) | Energyb/eV | ||
|---|---|---|---|---|
| LE | CT | LE | CT | |
| NNI-OH | 43.83 | 89.93 | -26471.24 | -26471.47 |
| NNI-OMe | 44.26 | 88.12 | -27540.53 | -27540.71 |
| NNI-NH2 | 47.19 | 90.04 | -25931.10 | -25930.92 |
Table 1 Dihedral angle and energy at optimized S1 of NNI-R
| Molecule | Dihedral anglea/(°) | Energyb/eV | ||
|---|---|---|---|---|
| LE | CT | LE | CT | |
| NNI-OH | 43.83 | 89.93 | -26471.24 | -26471.47 |
| NNI-OMe | 44.26 | 88.12 | -27540.53 | -27540.71 |
| NNI-NH2 | 47.19 | 90.04 | -25931.10 | -25930.92 |
| Molecule | d(N2—C3)/nm | ∠C1—N2—C3—C4/(°) | RMSD | ||||
|---|---|---|---|---|---|---|---|
| S0 | S1 | Δ(S0-S1) | S0 | S1 | Δ(S0-S1) | ||
| NNI-H | 0.14408 | 0.14399 | 0.00009 | 61.58 | 58.75 | 2.83 | 0.05 |
| NNI-Me | 0.14404 | 0.13706 | 0.00698 | 61.42 | 38.47 | 22.95 | 0.36 |
| NNI-tBu | 0.14401 | 0.13709 | 0.00692 | 61.34 | 38.59 | 22.75 | 0.36 |
| NNI-OH | 0.14393 | 0.13749 | 0.00644 | 61.17 | 39.06 | 22.11 | 0.29 |
| NNI-OMe | 0.14395 | 0.13762 | 0.00633 | 61.60 | 39.20 | 22.40 | 0.31 |
| NNI-NH2 | 0.14397 | 0.13848 | 0.00549 | 61.68 | 41.29 | 20.39 | 0.28 |
| NNI-NMe2 | 0.14396 | 0.13871 | 0.00525 | 62.02 | 41.52 | 20.50 | 0.34 |
Table 2 Bond length and dihedral angle between the donor and the acceptor, and the root mean square deviation(RMSD) at optimized S0 and S1 of NNI-R
| Molecule | d(N2—C3)/nm | ∠C1—N2—C3—C4/(°) | RMSD | ||||
|---|---|---|---|---|---|---|---|
| S0 | S1 | Δ(S0-S1) | S0 | S1 | Δ(S0-S1) | ||
| NNI-H | 0.14408 | 0.14399 | 0.00009 | 61.58 | 58.75 | 2.83 | 0.05 |
| NNI-Me | 0.14404 | 0.13706 | 0.00698 | 61.42 | 38.47 | 22.95 | 0.36 |
| NNI-tBu | 0.14401 | 0.13709 | 0.00692 | 61.34 | 38.59 | 22.75 | 0.36 |
| NNI-OH | 0.14393 | 0.13749 | 0.00644 | 61.17 | 39.06 | 22.11 | 0.29 |
| NNI-OMe | 0.14395 | 0.13762 | 0.00633 | 61.60 | 39.20 | 22.40 | 0.31 |
| NNI-NH2 | 0.14397 | 0.13848 | 0.00549 | 61.68 | 41.29 | 20.39 | 0.28 |
| NNI-NMe2 | 0.14396 | 0.13871 | 0.00525 | 62.02 | 41.52 | 20.50 | 0.34 |
| Molecule | NNI-H | NNI-Me | NNI-tBu | NNI-OH | NNI-OMe | NNI-NH2 | NNI-NMe2 |
|---|---|---|---|---|---|---|---|
| -0.3798 | -0.2452 | -0.2462 | -0.2110 | -0.2024 | -0.1658 | -0.1480 | |
| -1.5268 | -1.2703 | -1.2769 | -1.0584 | -1.0064 | -0.6765 | -0.5221 |
Table 3 Calculated adiabatic energy difference(ΔEST) between S1 and T1/T2 state of NNI-R
| Molecule | NNI-H | NNI-Me | NNI-tBu | NNI-OH | NNI-OMe | NNI-NH2 | NNI-NMe2 |
|---|---|---|---|---|---|---|---|
| -0.3798 | -0.2452 | -0.2462 | -0.2110 | -0.2024 | -0.1658 | -0.1480 | |
| -1.5268 | -1.2703 | -1.2769 | -1.0584 | -1.0064 | -0.6765 | -0.5221 |
| Molecule | Δ | ||||
|---|---|---|---|---|---|
| NNI-H | 0.66 | 0.87 | -0.160 | 0.016 | 0.023 |
| NNI-Me | 0.14 | 0.33 | 0.229 | 0.426 | 0.040 |
| NNI-tBu | 0.14 | 0.33 | 0.229 | 0.428 | 0.038 |
| NNI-OH | 0.13 | 0.31 | 0.256 | 0.451 | 0.040 |
| NNI-OMe | 0.12 | 0.30 | 0.262 | 0.461 | 0.037 |
| NNI-NH2 | 0.11 | 0.28 | 0.297 | 0.499 | 0.033 |
| NNI-NMe2 | 0.10 | 0.26 | 0.322 | 0.529 | 0.021 |
Table 4 Main parameters about the hole-electron distributions of NNI-R*
| Molecule | Δ | ||||
|---|---|---|---|---|---|
| NNI-H | 0.66 | 0.87 | -0.160 | 0.016 | 0.023 |
| NNI-Me | 0.14 | 0.33 | 0.229 | 0.426 | 0.040 |
| NNI-tBu | 0.14 | 0.33 | 0.229 | 0.428 | 0.038 |
| NNI-OH | 0.13 | 0.31 | 0.256 | 0.451 | 0.040 |
| NNI-OMe | 0.12 | 0.30 | 0.262 | 0.461 | 0.037 |
| NNI-NH2 | 0.11 | 0.28 | 0.297 | 0.499 | 0.033 |
| NNI-NMe2 | 0.10 | 0.26 | 0.322 | 0.529 | 0.021 |
| Molecule | Charge[substituent, (-R)]/C | Charge[donor moiety, (Ph+R)]/C | ||||
|---|---|---|---|---|---|---|
| S0 | S1 | S0→S1 | S0 | S1 | S0→S1 | |
| NNI-H | 0.2131 | 0.2126 | -0.0005 | 0.2590 | 0.2603 | 0.0013 |
| NNI-Me | 0.0432 | 0.0799 | 0.0367 | 0.2612 | 0.8103 | 0.5491 |
| NNI-tBu | 0.0192 | 0.0608 | 0.0416 | 0.2599 | 0.8111 | 0.5512 |
| NNI-OH | -0.2106 | -0.1212 | 0.0894 | 0.2605 | 0.8649 | 0.6044 |
| NNI-OMe | -0.2250 | -0.1135 | 0.1115 | 0.2616 | 0.8799 | 0.6183 |
| NNI-NH2 | -0.0566 | 0.1350 | 0.1916 | 0.2654 | 0.9456 | 0.6802 |
| NNI-NMe2 | -0.0553 | 0.1812 | 0.2365 | 0.2685 | 0.9712 | 0.7026 |
Table 5 Natural charge distribution of the substituents and the donor moieties atoptimized S0 and S1 state, and charge transfer from S0 to S1 state of NNI-R
| Molecule | Charge[substituent, (-R)]/C | Charge[donor moiety, (Ph+R)]/C | ||||
|---|---|---|---|---|---|---|
| S0 | S1 | S0→S1 | S0 | S1 | S0→S1 | |
| NNI-H | 0.2131 | 0.2126 | -0.0005 | 0.2590 | 0.2603 | 0.0013 |
| NNI-Me | 0.0432 | 0.0799 | 0.0367 | 0.2612 | 0.8103 | 0.5491 |
| NNI-tBu | 0.0192 | 0.0608 | 0.0416 | 0.2599 | 0.8111 | 0.5512 |
| NNI-OH | -0.2106 | -0.1212 | 0.0894 | 0.2605 | 0.8649 | 0.6044 |
| NNI-OMe | -0.2250 | -0.1135 | 0.1115 | 0.2616 | 0.8799 | 0.6183 |
| NNI-NH2 | -0.0566 | 0.1350 | 0.1916 | 0.2654 | 0.9456 | 0.6802 |
| NNI-NMe2 | -0.0553 | 0.1812 | 0.2365 | 0.2685 | 0.9712 | 0.7026 |
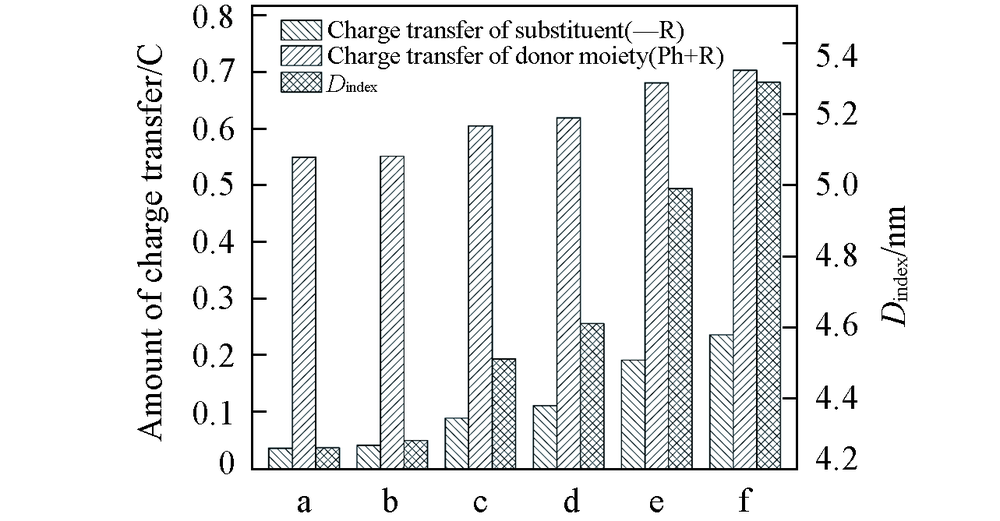
Fig.4 Charge change of substitutents and donor moieties during from S0 to S1 state and the change of Dindex a. NNI-Me; b. NNI-tBu; c. NNI-OH; d. NNI-OMe; e. NNI-NH2; f. NNI-NMe2.
| Molecule | ΔE/eV | f | 10-7 kFL/s-1 |
|---|---|---|---|
| NNI-H | 3.57 | 0.2697 | 14.7556 |
| NNI-Me | 2.91 | 0.0093 | 0.3369 |
| NNI-tBu | 2.92 | 0.0091 | 0.3321 |
| NNI-OH | 2.71 | 0.0090 | 0.2823 |
| NNI-OMe | 2.66 | 0.0086 | 0.2604 |
| NNI-NH2 | 2.37 | 0.0075 | 0.1805 |
| NNI-NMe2 | 2.28 | 0.0066 | 0.1473 |
Table 6 Calculated fluorescent rate(kFL), with the excitation energy(ΔE) and oscillator strength(f) of S1 state of NNI-R
| Molecule | ΔE/eV | f | 10-7 kFL/s-1 |
|---|---|---|---|
| NNI-H | 3.57 | 0.2697 | 14.7556 |
| NNI-Me | 2.91 | 0.0093 | 0.3369 |
| NNI-tBu | 2.92 | 0.0091 | 0.3321 |
| NNI-OH | 2.71 | 0.0090 | 0.2823 |
| NNI-OMe | 2.66 | 0.0086 | 0.2604 |
| NNI-NH2 | 2.37 | 0.0075 | 0.1805 |
| NNI-NMe2 | 2.28 | 0.0066 | 0.1473 |
| Molecule | NNI-Me | NNI-tBu | NNI-OH | NNI-OMe | NNI-NH2 | NNI-NMe2 |
|---|---|---|---|---|---|---|
| <S1|Hso| T2> | 0.6365 | 0.6769 | 0.4831 | 0.4576 | 2.2291 | 2.0871 |
| <S1|Hso| T1> | 3.1735 | 3.2833 | 2.7589 | 2.723 | 0.3552 | 0.3213 |
Table 7 Calculated spin-orbit coupling(SOC) matrix elements(cm-1) at optimized S1 geometry
| Molecule | NNI-Me | NNI-tBu | NNI-OH | NNI-OMe | NNI-NH2 | NNI-NMe2 |
|---|---|---|---|---|---|---|
| <S1|Hso| T2> | 0.6365 | 0.6769 | 0.4831 | 0.4576 | 2.2291 | 2.0871 |
| <S1|Hso| T1> | 3.1735 | 3.2833 | 2.7589 | 2.723 | 0.3552 | 0.3213 |
| Molecule | ΔEST/eV | λ/eV | VSOC/cm-1 | 10-7 kISC/s-1 | Molecule | ΔEST/eV | λ/eV | VSOC/cm-1 | 10-7 kISC/s-1 |
|---|---|---|---|---|---|---|---|---|---|
| NNI-Me | -1.2703 | 0.6740 | 3.1735 | 1.8778 | NNI-OMe | -1.0064 | 0.6559 | 2.7230 | 38.4418 |
| NNI-tBu | -1.2769 | 0.6704 | 3.2833 | 1.6393 | NNI-NH2 | -0.1658 | 0.1025 | 2.2291 | 275.8464 |
| NNI-OH | -1.0584 | 0.6612 | 2.7589 | 23.8786 | NNI-NMe2 | -0.1480 | 0.1716 | 2.0871 | 264.8364 |
Table 8 Calculated ISC rate(kISC) with the singlet-triplet energy gap(ΔEST), the reorganization energy(λ), and thespin-orbit coupling matrix element(VSOC)*
| Molecule | ΔEST/eV | λ/eV | VSOC/cm-1 | 10-7 kISC/s-1 | Molecule | ΔEST/eV | λ/eV | VSOC/cm-1 | 10-7 kISC/s-1 |
|---|---|---|---|---|---|---|---|---|---|
| NNI-Me | -1.2703 | 0.6740 | 3.1735 | 1.8778 | NNI-OMe | -1.0064 | 0.6559 | 2.7230 | 38.4418 |
| NNI-tBu | -1.2769 | 0.6704 | 3.2833 | 1.6393 | NNI-NH2 | -0.1658 | 0.1025 | 2.2291 | 275.8464 |
| NNI-OH | -1.0584 | 0.6612 | 2.7589 | 23.8786 | NNI-NMe2 | -0.1480 | 0.1716 | 2.0871 | 264.8364 |
| [1] | Li J. S., Zhou W. Y., Ouyang X. Y., Yu H., Yang R. H., Tan W. H., Yuan J. L., Anal. Chem., 2011, 83(4), 1356—1362 |
| [2] | Reineke S., Lindner F., Schwartz G., Seidler N., Walzer K., Lüssem B., Leo K., Nature, 2009, 459(7244), 234—239 |
| [3] | Sun Y. L., Giebink N. C., Kanno H., Ma B. W., Thompson M. E., Forrest S. R., Nature, 2006, 44(7086), 908—912 |
| [4] | Takeuchi T., Zhang S. J., Negishi K., Yoshihara T., Hosaka M., Iida Y., Tobita S., Cancer. Res., 2010, 70(11), 4490—4499 |
| [5] | Zhang G., Palmer G. M., Dewhirst M. W., Fraser C. L., Nat. Mater., 2009, 8(9), 747—751 |
| [6] | Baldo M. A., O'Brien D. F., You Y., Shoustikov A., Sibley S., Thompson M. E., Forrest S. R., Nature, 1998, 395(6698), 151—154 |
| [7] | Ma Y. G., Che C. M., Chao H. Y., Zhou X. M., Chan W. H., Shen J. C., Ad. Mater., 1999, 11(10), 852—857 |
| [8] | O’Brien D. F., Baldo M. A., Thompson M. E., Forrest S. R., App. Phys. Lett., 1999, 74(3), 442—444 |
| [9] | Baldo M. A., Lamansky S., Burrows P. E., Thompson M. E., Forrest S. R., App. Phys. Lett., 1999, 75(1), 4—6 |
| [10] | Lamansky S., Djurovich P., Murphy D., Abdel-Razzaq F., Lee H. E., Adachi C., Burrows P. E., Forrest S. R., Thompson M. E., J. Am. Chem, Soc., 2001, 123(18), 4304—4312 |
| [11] | Adachi C., Baldo M. A., Thompson M. E., Forrest S. R., J. Appl. Phys., 2001, 90(10), 5048—5051 |
| [12] | Pang X., Jin W. J ., Halogen Bonding in the Design of Organic Phosphors, Springer, Berlin, 2015, 115—146 |
| [13] | Lewis G. N., Kasha M., J. Am. Chem. Soc., 1944, 66(12), 2100—2116 |
| [14] | Reineke S., Baldo M. A., Sci. Rep., 2014, 4(1), 3797—3804 |
| [15] | Smith A. R. G., Burn P. L., Powell B. J., ChemPhysChem, 2011, 12(13), 2429—2438 |
| [16] | Yang Z. Y., Mao Z., Zhang X. P., Ou D. P., Mu Y. X., Zhang Y., Zhao C. Y., Liu S. W., Chi Z. G., Xu J. R., Angew. Chem. Int. Ed., 2016, 55(6), 2181—2225 |
| [17] | Seybold P. G., White W., Anal. Chem., 1975, 47(7), 1199—1200 |
| [18] | Yersin H., Rausch A. F., Czerwieniec R., Hofbeck T., Fischer T., Coord. Chem. Rev., 2011, 255(21), 2622—2652 |
| [19] | Rausch A. F., Homeier H. H., Djurovich P. I., Thompson M. E., Yersin H., Proceeding of SPIE, San Diego, 2007, 6655, 66550F |
| [20] | Chow P. K., Cheng G., Tong G. S. M., Ma C., Kwok W. M., Ang W. H., Chung C. Y., Yang C., Wang F., Che C. M., Chem. Sci., 2016, 7(9), 6083—6098 |
| [21] | Usuki T., Uchida H., Omoto K., Yamanoi Y., Yamada A., Iwamura M., Nozaki K., Nishihara H., J. Org. Chem., 2019, 84(17), 10749—10756 |
| [22] | Tao P., Li W. L., Zhang J., Guo S., Zhao Q., Wang H., Wei B., Liu S. J., Zhou X. H., Yu Q., Xu B. S., Huang W., Adv. Funct. Mater., 2016, 26(6), 881—894 |
| [23] | Kuei C. Y., Tsai W. L., Tong B. H., Jiao M., Lee W. K., Chi Y., Wu C. C., Liu S. H., Lee G. H., Chou P. T., Adv. Mater., 2016, 28(14), 2795—2800 |
| [24] | Lai P. N., Brysacz C. H., Alam M. K., Ayoub N. A., Gray T. G., Bao J., Teets T. S., J. Am. Chem. Soc., 2018, 140(32), 10198—10207 |
| [25] |
Brandt J. R., Wang X., Yang Y., Campbell A. J., Fuchter M. J., J. Am. Chem. Soc., 2016, 138(31), 9743—9746
doi: 10.1021/jacs.6b02463 URL |
| [26] | Shafikov M. Z., Daniels R., Kozhevnikov V. N., J. Phys. Chem. Lett., 2019, 10(22), 7015—7024 |
| [27] | Yan Z. M., Wang Y. P., Ding J. Q., Wang Y., Wang L. X., ACS Appl. Mater. Interfaces., 2018, 10(2), 1888—1896 |
| [28] | Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J., Heavy Metal Toxicity and the Environment, Springer, Berlin, 2012, 133—164 |
| [29] | Stohs S. J., Bagchi D., Free Radic. Bio. Med., 1995, 18(2), 321—336 |
| [30] | Jan A. T., Azam M., Siddiqui K., Ali A., Choi I., Haq Q. M., Int. J. Mol. Sci., 2015, 16(12), 29592—29630 |
| [31] | Braterman P. S., Cross R. J., Chem. Soc. Rev., 1973, 2(3), 271—294 |
| [32] | Wang Y., Zhang X. G., Han B., Peng J. B., Hou S. Y., Huang Y., Sun H. Q., Xie M. G., Lu Z. Y., Dyes Pigm., 2010, 86(2), 190—196 |
| [33] | Grabtchev I., Philipova T., Méallier P., Guittonneau S., Dyes Pigm., 1996, 31(1), 31—34 |
| [34] | Lee J. F., Hsu S. L. C., Polymer, 2009, 50(24), 5668—5674 |
| [35] | Cao H., Chang V., Hernandez R., J. Org. Chem., 2005, 70(13), 4929—4934 |
| [36] | Paudel S., Nandhikonda P., Heagy M. D. A., J. Fluoresc., 2009, 19(4), 681—691 |
| [37] |
Demeter A., Bérces T., Biczók L., Wintgens V., Valat P., Kossanyi J., J. Phys. Chem., 1996, 100(6), 2001—2011
doi: 10.1021/jp951133n URL |
| [38] | Hoa G. H., Kossanyi J., Demeter A., Biczok L., Berces T., Photochem. Photobiol. Sci., 2004, 3(5), 473—482 |
| [39] | Zhang X. P., Xie T. Q., Cui M. X., Yang L., Sun X. X., Jiang J., Zhang G. Q., ACS Appl. Mater. Inter., 2014, 6(4), 2279—2284 |
| [40] | Chen X. F., Xu C., Wang T., Zhou C., Du J. J., Wang Z. P., Xu H. X., Xie T. Q., Bi G. Q., Jiang J., Zhang X. P., Demas J. N., Trindle C. O., Luo Y., Zhang G. Q., Angew. Chem. Int. Ed., 2016, 55(34), 9872—9877 |
| [41] |
Liu R., Gao X., Barbatti M., Jiang J., Zhang G. Z., J. Phys. Chem. Lett., 2019, 10(6), 1388—1393
doi: 10.1021/acs.jpclett.9b00286 URL |
| [42] | Jacopo T., Benedetta M., Roberto C., Chem. Rev., 2005, 105(8), 2999—3093 |
| [43] | Gao X., Bai S. M., Daniele F., Thomas N., Mario B., Walter T., J. Chem. Theory Comput., 2017, 13(2), 515—524 |
| [44] | Ferré N., Filatov M., Huix-Rotllant M., Density-functional Methods for Excited States, Springer,Berlin, 2015 |
| [45] | Chai J. D., Martin H. G., Phys. Chem. Chem. Phys., 2008, 10(44), 6615—6620 |
| [46] | Hehre W. J., Ditchfield R., Pople J. A., J. Chem. Phys., 1972, 56(5), 2257—2261 |
| [47] | Francl M. M., Pietro W. J., Hehre W. J., Binkley J. S., Gordon M. S., J. Chem. Phys., 1982, 77(7), 3654—3665 |
| [48] | Cho D. W., Fujitsuka M., Sugimoto A., Yoon U. C., Mariano P. S., Majima T., J. Phys. Chem. B, 2006, 110(23), 11062—11068 |
| [49] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Petersson G. A., Nakatsuji H., Li X., Caricato M., Marenich A. V., Bloino J., Janesko B. G., Gomperts R., Mennucci B., Hratchian H. P., Ortiz J. V., Izmaylov A. F., Sonnenberg J. L., Williams-Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V. G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M. J., Heyd J. J., Brothers E. N., Kudin K. N., Staroverov V. N., Keith T. A., Kobayashi R., Normand J., Raghavachari K., Rendell A. P., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Millam J. M., Klene M., Adamo C., Cammi R., Ochterski J. W., Martin R. L., Morokuma K., Farkas O., Foresman J. B., Fox D. J., Gaussian 16, Rev.B.01, Gaussian Inc., Wallingford CT, 2016 |
| [50] | Zhao Y., Truhlar D. G., J. Phys. Chem., 2006, 110(49), 13126—13130 |
| [51] | Zhao Y., Truhlar D. G., Theor. Chem. Acc., 2008, 120(1), 215—241 |
| [52] | Zhao Y., Truhlar D. G., J. Chem. Phys., 2006, 125(19), 194101—194118 |
| [53] |
Grimme S., Antony J., Ehrlich S., Krieg H., J. Chem. Phys., 2010, 132(15), 154104—154122
doi: 10.1063/1.3382344 URL |
| [54] | Grimme S., Ehrlich S., Goerigk L., J. Comput. Chem., 2011, 32(7), 1456—1465 |
| [55] | Lu T., Chen F. W., J. Comput. Chem., 2012, 33(5), 580—592 |
| [56] | Chiodo S. G., Russo N., J. Comput. Chem., 2009, 30(5), 832—839 |
| [57] |
Duan Y. C., Wen L. L., Gao Y., Wu Y., Zhao L., Geng Y., Shan G. G., Zhang M., Su Z. M., J. Phys. Chem. C, 2018, 122(40), 23091—23101
doi: 10.1021/acs.jpcc.8b06533 URL |
| [58] | Beljonne D., Shuai Z., Pourtois G., Bredas J. L., J. Phys. Chem. A, 2001, 105(15), 3899—3907 |
| [1] | 李欣宇, 李志伟, 张兴元. 硫磺素型聚乳酸/苯磺酸室温磷光体系的构建[J]. 高等学校化学学报, 2021, 42(6): 1987. |
| [2] | 房晓星, 郑吉, 闫桂琴. 量子点室温磷光探针检测生物体液中的头孢哌酮钠舒巴坦钠[J]. 高等学校化学学报, 2016, 37(8): 1435. |
| [3] | 戎佳萌, 周操, 徐栋, 孙伟, 黄晓雯, 张兴元. 基于二苯甲酮衍生物的端羟基聚乳酸的双重发光性能[J]. 高等学校化学学报, 2016, 37(8): 1542. |
| [4] | 李毓骐,朱亚先,张勇 . 银纳米粒子的绿色合成及其对荧光素室温磷光的增强效应[J]. 高等学校化学学报, 2008, 29(4): 669. |
| [5] | 吴娅兰, 李隆弟, 刘佳铭, 朱国辉. CaCl2作为异硫氰酸曙红固体基质室温磷光增强剂及其在免疫分析中的应用[J]. 高等学校化学学报, 2005, 26(1): 49. |
| [6] | 李隆弟, 吴应光, 童爱军, 龙文清. 光敏离子载体丹磺酰基-单氮杂-18-冠-6的阳离子识别性质研究[J]. 高等学校化学学报, 2001, 22(9): 1472. |
| [7] | 袁雯, 晋卫军, 董川. 水介质钯卟啉室温磷光探针与小牛胸腺DNA作用的光谱特性[J]. 高等学校化学学报, 2001, 22(6): 922. |
| [8] | 刘佳铭, 付艳, 余冰宾, 李隆弟. 固体基质室温磷光免疫分析法测定人IgG[J]. 高等学校化学学报, 2001, 22(10): 1645. |
| [9] | 李隆弟, 牟兰, 陈小康. 多环芳烃芴、苊的无保护流体室温磷光性质研究[J]. 高等学校化学学报, 2000, 21(7): 1040. |
| [10] | 陈小康, 牟兰, 李隆弟. α-萘氧乙酸无保护流体室温磷光的重原子效应及有机溶剂的影响[J]. 高等学校化学学报, 1999, 20(7): 1052. |
| [11] | 牟兰, 陈小康, 李隆弟. β-溴代萘的无保护流体室温磷光性质及介质效应[J]. 高等学校化学学报, 1999, 20(2): 214. |
| [12] | 张勇, 朱亚先, 杜新贞, 黄贤智, 陈国珍. 动力学室温磷光法研究外磁场效应对不同脂肪醇/α-溴代萘/β-环糊精包络物生成速度的影响[J]. 高等学校化学学报, 1998, 19(1): 39. |
| [13] | 杜新贞, 张勇, 江云宝, 林丽榕, 黄贤智, 陈国珍. β-环糊精存在下直链脂肪醇诱导1-溴萘室温磷光光谱研究[J]. 高等学校化学学报, 1997, 18(12): 1935. |
| [14] | 杨欣, 董川, 魏雁声, 晋卫军, 刘长松. 同步扫描-微乳状液增稳室温磷光法同时测定痕量多环芳烃的研究[J]. 高等学校化学学报, 1996, 17(5): 716. |
| [15] | 杨欣, 董川, 晋卫军, 魏雁声, 刘长松. 同步-导数-化学除氧微乳状液增稳室温磷光法测定痕量荧蒽与屈的研究[J]. 高等学校化学学报, 1996, 17(4): 547. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||