

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (2): 228.doi: 10.7503/cjcu20190605
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
收稿日期:2019-11-25
出版日期:2020-02-10
发布日期:2019-12-31
通讯作者:
宋大千
E-mail:songdq@jlu.edu.cn
基金资助:
PIAO Huilan,MA Pinyi,QIN Zucheng,JIANG Yanxiao,SUN Ying,WANG Xinghua,SONG Daqian( )
)
Received:2019-11-25
Online:2020-02-10
Published:2019-12-31
Contact:
Daqian SONG
E-mail:songdq@jlu.edu.cn
Supported by:摘要:
开发了一种高效、 环保的基于酸性离子液体填充注射器的泡腾辅助微萃取法, 用于测定果汁样品中三嗪类除草剂. 萃取分散采用酸性离子液体[C4mim][HSO4], 它对三嗪类除草剂具有较高的溶解度, 其酸性可与碳酸盐反应产生二氧化碳, 从而加速萃取过程. 该实验的提取和分离步骤在注射器中完成, 整个预处理过程完全不需要任何设备辅助. 对碳酸氢钠用量、 酸性离子液体用量、 盐添加量及洗脱溶剂体积等影响萃取效率的实验条件进行了优化. 在最佳条件下, 三嗪类除草剂浓度在1~200 ng/mL范围内获得了良好的线性关系, 相关系数大于0.9984; 检出限(LOD)和定量限(LOQ)分别为0.06~0.18和0.21~0.61 ng/mL, 日间及日内精密度低于8.3%. 实验结果表明, 该方法可用于果汁样品中三嗪类除草剂的测定.
中图分类号:
TrendMD:
朴惠兰,马品一,覃祖成,姜延晓,孙颖,王兴华,宋大千. 基于酸性离子液体填充注射器的泡腾辅助微萃取法测定果汁样品中三嗪类除草剂. 高等学校化学学报, 2020, 41(2): 228.
PIAO Huilan,MA Pinyi,QIN Zucheng,JIANG Yanxiao,SUN Ying,WANG Xinghua,SONG Daqian. Determination of Triazine Herbicides from Fruit Juice Samples Using Effervescence Assisted Microextraction Method Based on Acidic Ionic Liquid Packed Syringe. Chem. J. Chinese Universities, 2020, 41(2): 228.
| Analyte | Liner range/ (ng·mL-1) | Regression equation | Correlation coefficient, r | LOD/ (ng·mL-1) | LOQ/ (ng·mL-1) | RSD(%) | |
|---|---|---|---|---|---|---|---|
| Inter-day | Intra-day | ||||||
| Atraton | 1—200 | A=32273c-45035 | 0.9993 | 0.06 | 0.21 | 1.6 | 7.6 |
| Desmetryn | 1—200 | A=22833c-32310 | 0.9991 | 0.09 | 0.31 | 6.8 | 7.2 |
| Secbumeton | 2—200 | A=18533c-30871 | 0.9984 | 0.16 | 0.54 | 5.8 | 7.8 |
| Terbumeton | 2—200 | A=16835c-24740 | 0.9990 | 0.18 | 0.61 | 5.4 | 6.9 |
| Prometryn | 1—200 | A=26847c-34238 | 0.9990 | 0.06 | 0.18 | 2.9 | 6.3 |
Table 1 Analytical performances of the present method
| Analyte | Liner range/ (ng·mL-1) | Regression equation | Correlation coefficient, r | LOD/ (ng·mL-1) | LOQ/ (ng·mL-1) | RSD(%) | |
|---|---|---|---|---|---|---|---|
| Inter-day | Intra-day | ||||||
| Atraton | 1—200 | A=32273c-45035 | 0.9993 | 0.06 | 0.21 | 1.6 | 7.6 |
| Desmetryn | 1—200 | A=22833c-32310 | 0.9991 | 0.09 | 0.31 | 6.8 | 7.2 |
| Secbumeton | 2—200 | A=18533c-30871 | 0.9984 | 0.16 | 0.54 | 5.8 | 7.8 |
| Terbumeton | 2—200 | A=16835c-24740 | 0.9990 | 0.18 | 0.61 | 5.4 | 6.9 |
| Prometryn | 1—200 | A=26847c-34238 | 0.9990 | 0.06 | 0.18 | 2.9 | 6.3 |
| Matrix | Spiked/ (ng·mL-1) | Atraton | Desmetryn | Secbumeton | Terbumeton | Prometryn | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Fruit Juice | 3.0 | 103.4 | 7.2 | 101.6 | 7.3 | 114.9 | 4.7 | 102.5 | 6.0 | 94.3 | 4.7 |
| 10.0 | 102.4 | 4.6 | 104.0 | 5.8 | 107.7 | 6.5 | 83.0 | 5.3 | 123.2 | 8.2 | |
| 20.0 | 106.0 | 2.0 | 105.0 | 2.2 | 96.6 | 1.5 | 89.6 | 3.6 | 116.5 | 5.8 | |
| Tea beverage | 3.0 | 90.4 | 6.9 | 123.5 | 4.4 | 107.6 | 8.7 | 103.2 | 4.0 | 94.4 | 4.6 |
| 10.0 | 91.5 | 2.6 | 101.0 | 5.3 | 100.6 | 2.7 | 85.2 | 3.4 | 100.3 | 6.1 | |
| 20.0 | 96.9 | 5.7 | 119.0 | 6.0 | 112.5 | 6.0 | 93.1 | 5.7 | 100.1 | 6.2 | |
| Water | 3.0 | 104.9 | 8.3 | 118.3 | 5.3 | 112.5 | 8.3 | 83.9 | 2.3 | 109.1 | 4.3 |
| 10.0 | 96.5 | 2.2 | 102.1 | 7.1 | 99.7 | 7.9 | 80.8 | 7.2 | 104.0 | 5.3 | |
| 20.0 | 92.1 | 0.4 | 112.3 | 1.5 | 101.4 | 1.5 | 94.9 | 3.6 | 113.8 | 3.0 | |
Table 2 Analytical results of real fruit juice samples
| Matrix | Spiked/ (ng·mL-1) | Atraton | Desmetryn | Secbumeton | Terbumeton | Prometryn | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Fruit Juice | 3.0 | 103.4 | 7.2 | 101.6 | 7.3 | 114.9 | 4.7 | 102.5 | 6.0 | 94.3 | 4.7 |
| 10.0 | 102.4 | 4.6 | 104.0 | 5.8 | 107.7 | 6.5 | 83.0 | 5.3 | 123.2 | 8.2 | |
| 20.0 | 106.0 | 2.0 | 105.0 | 2.2 | 96.6 | 1.5 | 89.6 | 3.6 | 116.5 | 5.8 | |
| Tea beverage | 3.0 | 90.4 | 6.9 | 123.5 | 4.4 | 107.6 | 8.7 | 103.2 | 4.0 | 94.4 | 4.6 |
| 10.0 | 91.5 | 2.6 | 101.0 | 5.3 | 100.6 | 2.7 | 85.2 | 3.4 | 100.3 | 6.1 | |
| 20.0 | 96.9 | 5.7 | 119.0 | 6.0 | 112.5 | 6.0 | 93.1 | 5.7 | 100.1 | 6.2 | |
| Water | 3.0 | 104.9 | 8.3 | 118.3 | 5.3 | 112.5 | 8.3 | 83.9 | 2.3 | 109.1 | 4.3 |
| 10.0 | 96.5 | 2.2 | 102.1 | 7.1 | 99.7 | 7.9 | 80.8 | 7.2 | 104.0 | 5.3 | |
| 20.0 | 92.1 | 0.4 | 112.3 | 1.5 | 101.4 | 1.5 | 94.9 | 3.6 | 113.8 | 3.0 | |
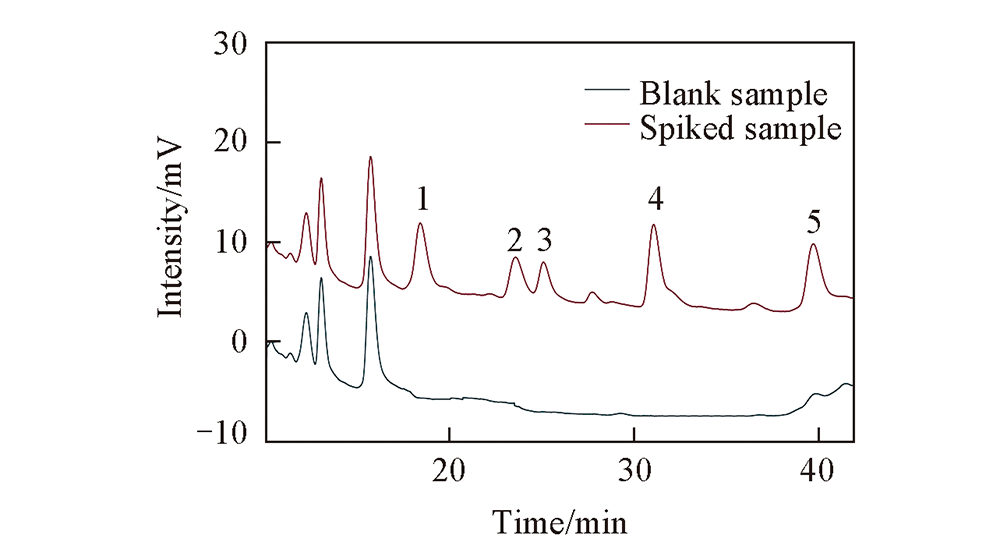
Fig.6 HPLC chromatograms of blank and spiked fruit juice samples Peak 1: atraton; peak 2: desmetryn; peak 3: secbumeton; peak 4: terbumeton; peak 5: prometryn.
| Matrix | Analytes | Extraction method | Detection | LOD/(ng·mL-1) | Recovery(%) | Ref. |
|---|---|---|---|---|---|---|
| Water | Triazine herbicides | SPME | HPLC-DAD | 0.05—0.2 | 86.0—94.6 | [ |
| Water | Triazine herbicides | HF-LLLME | HPLC-DAD | 0.07—0.69 | 85.2—113.0 | [ |
| Fruit juice | Triazine herbicides | CSDF-ME | HPLC-UV | 0.5—1.0 | 71.0—90.0 | [ |
| Milk | Triazine herbicides | MSPE | HPLC-DAD | 0.134—0.176 | 81.0—109.0 | [ |
| Milk | Triazine herbicides | SPE | HPLC-MS | 0.03—0.12 | 82.5—97.5 | [ |
| Water | Pesticide residues | UA-DLLME | GC-FID | 0.09—0.57 | 90.5—107.7 | [ |
| Fruit juice | Triazine herbicides | EA-DLLME | HPLC-UV | 0.06—0.18 | 80.8—123.5 | This work |
Table 3 Comparison of the present method with other reported methods
| Matrix | Analytes | Extraction method | Detection | LOD/(ng·mL-1) | Recovery(%) | Ref. |
|---|---|---|---|---|---|---|
| Water | Triazine herbicides | SPME | HPLC-DAD | 0.05—0.2 | 86.0—94.6 | [ |
| Water | Triazine herbicides | HF-LLLME | HPLC-DAD | 0.07—0.69 | 85.2—113.0 | [ |
| Fruit juice | Triazine herbicides | CSDF-ME | HPLC-UV | 0.5—1.0 | 71.0—90.0 | [ |
| Milk | Triazine herbicides | MSPE | HPLC-DAD | 0.134—0.176 | 81.0—109.0 | [ |
| Milk | Triazine herbicides | SPE | HPLC-MS | 0.03—0.12 | 82.5—97.5 | [ |
| Water | Pesticide residues | UA-DLLME | GC-FID | 0.09—0.57 | 90.5—107.7 | [ |
| Fruit juice | Triazine herbicides | EA-DLLME | HPLC-UV | 0.06—0.18 | 80.8—123.5 | This work |
| [1] |
Chen P. S., Haung W. Y., Huang S. D., J. Chromatogr. B, 2014,955/956, 116— 123
doi: 10.1016/j.jchromb.2014.02.032 URL |
| [2] |
Zhang W., Ruan G., Li X., Jiang X., Huang Y., Du F., Li J., Anal. Chim. Acta, 2019,1071, 17— 24
doi: 10.1016/j.aca.2019.04.041 URL |
| [3] |
Fang R., Chen G. H., Yi L. X., Shao Y. X., Zhang L., Cai Q. H., Xiao J., Food Chem., 2014,145, 41— 48
doi: 10.1016/j.foodchem.2013.08.028 URL |
| [4] | Chen L., Song D., Tian Y., Ding L., Yu A., Zhang H ., Tr AC-Trend. Anal. Chem., 2008,27, 151— 159 |
| [5] |
Qiao C., Bi S., Sun Y., Song D., Zhang H., Zhou W ., Spectrochim. Acta A, 2008,70, 136— 143
doi: 10.1016/j.saa.2007.07.038 URL |
| [6] |
Jiang Y., Piao H., Qin Z., Li X., Ma P., Sun Y., Wang X., Song D ., J. Sep. Sci., 2019,42, 2900— 2908
doi: 10.1002/jssc.v42.18 URL |
| [7] |
Sun T., Wang M., Wang D., Du Z ., Talanta, 2020,207, 120244
doi: 10.1016/j.talanta.2019.120244 URL |
| [8] |
Torbati M., Farajzadeh M. A., Mogaddam M. R. A., Torbati M., J. Sep. Sci., 2019,42, 1768— 1776
doi: 10.1002/jssc.v42.9 URL |
| [9] |
Guinez M., Canales R., Talio C., Gomez D., Smichowski P ., Talanta, 2020,206, 120182
doi: 10.1016/j.talanta.2019.120182 URL |
| [10] |
Rodríguez-González N., González-Castro M. J., Beceiro-González E., Muniategui-Lorenzo S., Microchem. J., 2017,133, 137— 143
doi: 10.1016/j.microc.2017.03.022 URL |
| [11] |
Yuan J., Cui Z., Cheng C., Wang X., Wang S., Song X., Li F., Acta Chromatogr., 2017,29, 487— 492
doi: 10.1556/1326.2016.00122 URL |
| [12] |
Camino-Sanchez F. J., Rodriguez-Gomez R., Zafra-Gomez A., Santos-Fandila A., Vilchez J. L., Talanta, 2014,130, 388— 399
doi: 10.1016/j.talanta.2014.07.022 URL |
| [13] |
Abujaber F., Guzman Bernardo F. J., Rodriguez Martin-Doimeadios R. C., Talanta, 2019,201, 266— 270
doi: 10.1016/j.talanta.2019.04.005 URL |
| [14] | Lasarte-Aragones G., Lucena R., Cardenas S., Valcarcel M., Anal. Chim. Acta, 2014,807, 61— 66 |
| [15] |
Wei Q., Song Z., Nie J., Xia H., Chen F., Li Z., Lee M ., J. Sep. Sci., 2016,39, 4603— 4609
doi: 10.1002/jssc.201600619 URL |
| [16] |
Ghoochani Moghadam A., Rajabi M., Hemmati M., Asghari A ., J. Mol. Liq., 2017,242, 1176— 1183
doi: 10.1016/j.molliq.2017.07.038 URL |
| [17] |
Yao L., Liu H., Wang X., Xu W., Zhu Y., Wang H., Pang L., Lin C., Food Chem., 2018,256, 212— 218
doi: 10.1016/j.foodchem.2018.02.132 URL |
| [18] |
Paduszyński K., Królikowski M., Orzeł P ., J. Mol. Liq., 2019,279, 733— 739
doi: 10.1016/j.molliq.2019.01.149 URL |
| [19] |
Wu X., Li X., Yang M., Zeng H., Zhang S., Lu R., Gao H., Xu D ., J. Chromatogr. A, 2017,1497, 1— 8
doi: 10.1016/j.chroma.2017.03.005 URL |
| [20] |
Pan H., Li H., Zhang H., Wang A., Yang S ., Fuel, 2019,239, 886— 895
doi: 10.1016/j.fuel.2018.11.093 URL |
| [21] | Guan L., Luo Q., Liang N., Yu W., Chem. J. Chinese Universities, 2018,34(6), 887— 892 |
| [22] |
Mehrdad A., Noorani N., Sep. Purif. Technol., 2019,226, 138— 145
doi: 10.1016/j.seppur.2019.05.086 URL |
| [23] |
Kong J., Zhu F., Huang W., He H., Hu J., Sun C., Xian Q., Yang S ., J. Chromatogr. A, 2019,1603, 92— 101
doi: 10.1016/j.chroma.2019.06.063 URL |
| [24] |
Farajzadeh M. A., Abbaspour M., Kazemian R., J. Chromatogr. A, 2019,1603, 51— 60
doi: 10.1016/j.chroma.2019.06.051 URL |
| [25] |
Wu Q., Feng C., Zhao G., Wang C., Wang Z ., J. Sep. Sci., 2012,35, 193— 199
doi: 10.1002/jssc.v35.2 URL |
| [26] |
Yang Q., Chen B., He M., Hu B ., Talanta, 2018,186, 88— 96
doi: 10.1016/j.talanta.2018.04.012 URL |
| [27] |
Ahmadi-Jouibari T., Pasdar Y., Pirsaheb M., Fattahi N ., Anal. Methods, 2017,9, 980— 985
doi: 10.1039/C6AY02839J URL |
| [28] |
Mohd N. I., Gopal K., Raoov M., Mohamad S., Yahaya N., Lim V., Zain N. N. M., Talanta, 2019,196, 217— 225
doi: 10.1016/j.talanta.2018.12.043 URL |
| [29] |
Zhang F., Zhao Q., Yan X., Li H., Zhang P., Wang L., Zhou T., Li Y., Ding L., Food Chem., 2016,197, 943— 949
doi: 10.1016/j.foodchem.2015.11.056 URL |
| [30] |
Cui S., Chen Q., Wang W., Miao J., Wang A., Chen J ., Chromatogr., 2013,76, 671— 678
doi: 10.1007/s10337-013-2441-7 URL |
| [1] | 刘强, 李贺, 张永红, 王斌, 孙亚栋, 阿布力米提·阿布都卡德, 刘晨江. Brönsted酸性离子液体催化芳香醛和2-甲基喹啉反应合成1,3-二(2-喹啉基)丙烷化合物[J]. 高等学校化学学报, 2015, 36(9): 1702. |
| [2] | 泮丽亚, 李志峰, 倪宇翔, 姚振刚, 余志平, 吴文康, 应安国. 新型酸性离子液体催化的 Knoevenagel缩合反应[J]. 高等学校化学学报, 2015, 36(1): 81. |
| [3] | 余传继, 刘晨江. BrΦnsted酸性离子液体[HSO3-bpy][HSO4]催化合成β-吲哚酮[J]. 高等学校化学学报, 2010, 31(6): 1158. |
| [4] | 职慧珍, 罗军, 马伟, 吕春绪. PEG型酸性温控离子液体中芳香酸和醇的酯化反应[J]. 高等学校化学学报, 2008, 29(4): 772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||