

Chem. J. Chinese Universities ›› 2025, Vol. 46 ›› Issue (7): 20240535.doi: 10.7503/cjcu20240535
• Physical Chemistry • Previous Articles Next Articles
WANG Zhiyuan, DONG Yi, QI Baohui, WEI Xueyang, ZHANG Jiahui, HUANG Qizhong, LI Jisheng, GAO Na, DI Shiying, HU Yufeng( )
)
Received:2024-12-09
Online:2025-07-10
Published:2025-01-18
Contact:
HU Yufeng
E-mail:huyf3581@sina.com
Supported by:CLC Number:
TrendMD:
WANG Zhiyuan, DONG Yi, QI Baohui, WEI Xueyang, ZHANG Jiahui, HUANG Qizhong, LI Jisheng, GAO Na, DI Shiying, HU Yufeng. Determination of Acidic Ionic Liquid H0 and the Effect of Salt Effect[J]. Chem. J. Chinese Universities, 2025, 46(7): 20240535.
| Cation | Abbreviation | Structure | Anion | Abbreviation | Structure |
|---|---|---|---|---|---|
| 2⁃Pyrrolidone cation | [HNHP]+ | 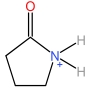 | Hydrogen sulfate anion | [HSO4] - | 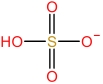 |
| N⁃Methylpyrrolidone cation | [HNMP]+ | 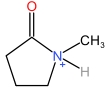 | Dihydrophosphate anion | [H2PO4] - | 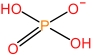 |
| N⁃Octylpyrrolidone cation | [HNOP]+ | 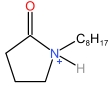 | Methanesulfonate anion | [MSA]- | 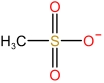 |
| N⁃Cyclohexylpyrrolidonecation | [HNCYP]+ | 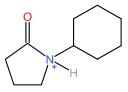 | Trifluoromethane sulfonate anion | [TFO]- | 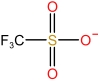 |
| 1⁃Methylimidazole cation | [MIM]+ | 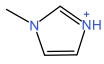 | p⁃Toluenesulfonic acid anion | [p⁃TSA]- | 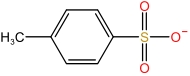 |
| 1⁃Propylsulfonic acid⁃3⁃methylimidazole cation | [C3SMIM]+ | 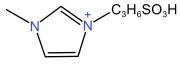 | p⁃Chlorophenylsulfonate anion | [p⁃ClBSA]- | 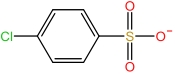 |
Table 1 Cations and anions of the ionic liquid used in this study
| Cation | Abbreviation | Structure | Anion | Abbreviation | Structure |
|---|---|---|---|---|---|
| 2⁃Pyrrolidone cation | [HNHP]+ |  | Hydrogen sulfate anion | [HSO4] - |  |
| N⁃Methylpyrrolidone cation | [HNMP]+ |  | Dihydrophosphate anion | [H2PO4] - |  |
| N⁃Octylpyrrolidone cation | [HNOP]+ |  | Methanesulfonate anion | [MSA]- |  |
| N⁃Cyclohexylpyrrolidonecation | [HNCYP]+ |  | Trifluoromethane sulfonate anion | [TFO]- |  |
| 1⁃Methylimidazole cation | [MIM]+ |  | p⁃Toluenesulfonic acid anion | [p⁃TSA]- |  |
| 1⁃Propylsulfonic acid⁃3⁃methylimidazole cation | [C3SMIM]+ |  | p⁃Chlorophenylsulfonate anion | [p⁃ClBSA]- |  |
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 1.154 | 100.00 | 0 | — |
| [HNHP][H2PO4] | 1.095 | 94.89 | 5.11 | 2.26 |
| [HNHP][MSA] | 1.067 | 92.46 | 7.54 | 2.08 |
| [HNHP][TFO] | 1.061 | 91.94 | 8.06 | 2.05 |
| [HNHP][HSO4] | 1.008 | 87.35 | 12.65 | 1.83 |
| [HNMP][MSA] | 1.056 | 91.51 | 8.49 | 2.02 |
| [HNMP][p⁃TSA] | 1.053 | 91.25 | 8.75 | 2.01 |
| [HNMP][p⁃ClBSA] | 1.039 | 90.03 | 9.97 | 1.95 |
| [HNMP][TFO] | 1.010 | 87.52 | 12.48 | 1.84 |
| [HNMP][HSO4] | 0.983 | 85.18 | 14.82 | 1.75 |
| [HNOP][H2PO4] | 0.834 | 72.27 | 27.73 | 1.41 |
| [HNOP][TFO] | 0.801 | 69.41 | 30.59 | 1.35 |
| [HNOP][HSO4] | 0.725 | 62.82 | 37.18 | 1.22 |
| [HNCYP][TFO] | 1.071 | 92.81 | 7.19 | 2.10 |
| [HNCYP][p⁃TSA] | 1.106 | 95.84 | 4.16 | 2.35 |
Table 2 H0 of pyrrolidone, N-methyl pyrrolidone, N-octylpyrrolidone and N-cyclohexyl-2-pyrrolidone ionic liquids
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 1.154 | 100.00 | 0 | — |
| [HNHP][H2PO4] | 1.095 | 94.89 | 5.11 | 2.26 |
| [HNHP][MSA] | 1.067 | 92.46 | 7.54 | 2.08 |
| [HNHP][TFO] | 1.061 | 91.94 | 8.06 | 2.05 |
| [HNHP][HSO4] | 1.008 | 87.35 | 12.65 | 1.83 |
| [HNMP][MSA] | 1.056 | 91.51 | 8.49 | 2.02 |
| [HNMP][p⁃TSA] | 1.053 | 91.25 | 8.75 | 2.01 |
| [HNMP][p⁃ClBSA] | 1.039 | 90.03 | 9.97 | 1.95 |
| [HNMP][TFO] | 1.010 | 87.52 | 12.48 | 1.84 |
| [HNMP][HSO4] | 0.983 | 85.18 | 14.82 | 1.75 |
| [HNOP][H2PO4] | 0.834 | 72.27 | 27.73 | 1.41 |
| [HNOP][TFO] | 0.801 | 69.41 | 30.59 | 1.35 |
| [HNOP][HSO4] | 0.725 | 62.82 | 37.18 | 1.22 |
| [HNCYP][TFO] | 1.071 | 92.81 | 7.19 | 2.10 |
| [HNCYP][p⁃TSA] | 1.106 | 95.84 | 4.16 | 2.35 |
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| m⁃Nitroaniline | 1.519 | 100.00 | 0 | — |
| [MIM][H2PO4] | 1.504 | 98.99 | 1.01 | 4.49 |
| [MIM][MSA] | 1.488 | 97.96 | 2.04 | 4.24 |
| [MIM][TFO] | 1.489 | 98.00 | 2.00 | 4.19 |
| [MIM][HSO4] | 1.447 | 95.23 | 4.77 | 3.86 |
| p⁃Nitroaniline | 1.061 | 100.00 | 0 | — |
| [C3SMIM][MSA] | 0.981 | 92.46 | 7.54 | 2.08 |
| [C3SMIM][TFO] | 0.974 | 91.80 | 8.20 | 2.04 |
| [C3SMIM][p⁃TSA] | 0.915 | 86.24 | 13.76 | 1.79 |
| [C3SMIM][HSO4] | 0.914 | 86.15 | 13.85 | 1.78 |
Table 3 H0 of imidazolium-based ionic liquids
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| m⁃Nitroaniline | 1.519 | 100.00 | 0 | — |
| [MIM][H2PO4] | 1.504 | 98.99 | 1.01 | 4.49 |
| [MIM][MSA] | 1.488 | 97.96 | 2.04 | 4.24 |
| [MIM][TFO] | 1.489 | 98.00 | 2.00 | 4.19 |
| [MIM][HSO4] | 1.447 | 95.23 | 4.77 | 3.86 |
| p⁃Nitroaniline | 1.061 | 100.00 | 0 | — |
| [C3SMIM][MSA] | 0.981 | 92.46 | 7.54 | 2.08 |
| [C3SMIM][TFO] | 0.974 | 91.80 | 8.20 | 2.04 |
| [C3SMIM][p⁃TSA] | 0.915 | 86.24 | 13.76 | 1.79 |
| [C3SMIM][HSO4] | 0.914 | 86.15 | 13.85 | 1.78 |
| Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution | Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution |
|---|---|---|---|---|---|
| [MIM][H2PO4] | 4.49 | 2.55 | [C3SMIM][HSO4] | 1.78 | 0.34 |
| [MIM][HSO4] | 3.86 | 0.73 | [HNMP][p⁃ClBSA] | 1.95 | 0.71 |
| [C3SMIM][TFO] | 2.04 | 0.01 | [HNMP][HSO4] | 1.75 | 0.52 |
Table 4 H0 of acidic ionic liquids in aqueous solution and dichloromethane solution
| Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution | Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution |
|---|---|---|---|---|---|
| [MIM][H2PO4] | 4.49 | 2.55 | [C3SMIM][HSO4] | 1.78 | 0.34 |
| [MIM][HSO4] | 3.86 | 0.73 | [HNMP][p⁃ClBSA] | 1.95 | 0.71 |
| [C3SMIM][TFO] | 2.04 | 0.01 | [HNMP][HSO4] | 1.75 | 0.52 |
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| CH3SO3H | 0.330 | 42.57 | 57.43 | 0.860 |
| CH3SO3H+NaCl | 0.319 | 41.12 | 58.88 | 0.834 |
| CH3SO3H+MgCl2 | 0.299 | 38.58 | 61.42 | 0.788 |
| CH3SO3H+C3SMIM | 0.414 | 53.39 | 46.61 | 1.049 |
| CH3SO3H+CF3SO3Na | 0.311 | 40.12 | 59.88 | 0.816 |
| CH3SO3H+CH3SO3Na | 0.322 | 41.45 | 58.55 | 0.840 |
| CH3SO3H+C3SMIM+CH3SO3Na | 0.361 | 46.49 | 53.51 | 0.929 |
| CH3SO3H+C3SMIM+CF3SO3Na | 0.335 | 43.14 | 56.86 | 0.870 |
| CF3SO3H | 0.325 | 41.90 | 58.10 | 0.848 |
| CF3SO3H+C3SMIM | 0.354 | 45.64 | 54.36 | 0.914 |
| CF3SO3H+CF3SO3Na | 0.306 | 39.40 | 60.60 | 0.803 |
| CF3SO3H+CF3SO3Na+C3SMIM | 0.332 | 42.74 | 57.26 | 0.863 |
Table 5 H0 of aqueous solution systems of CH3SO3H/CF3SO3H+salt
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| CH3SO3H | 0.330 | 42.57 | 57.43 | 0.860 |
| CH3SO3H+NaCl | 0.319 | 41.12 | 58.88 | 0.834 |
| CH3SO3H+MgCl2 | 0.299 | 38.58 | 61.42 | 0.788 |
| CH3SO3H+C3SMIM | 0.414 | 53.39 | 46.61 | 1.049 |
| CH3SO3H+CF3SO3Na | 0.311 | 40.12 | 59.88 | 0.816 |
| CH3SO3H+CH3SO3Na | 0.322 | 41.45 | 58.55 | 0.840 |
| CH3SO3H+C3SMIM+CH3SO3Na | 0.361 | 46.49 | 53.51 | 0.929 |
| CH3SO3H+C3SMIM+CF3SO3Na | 0.335 | 43.14 | 56.86 | 0.870 |
| CF3SO3H | 0.325 | 41.90 | 58.10 | 0.848 |
| CF3SO3H+C3SMIM | 0.354 | 45.64 | 54.36 | 0.914 |
| CF3SO3H+CF3SO3Na | 0.306 | 39.40 | 60.60 | 0.803 |
| CF3SO3H+CF3SO3Na+C3SMIM | 0.332 | 42.74 | 57.26 | 0.863 |
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| o⁃Nitroaniline | 2.828 | 100.00 | 0 | — |
| H2SO4+Na2HPO4 | 2.797 | 98.90 | 1.10 | 1.665 |
| H2SO4+NaH2PO4 | 2.646 | 93.56 | 6.44 | 0.873 |
| H2SO4+Na2SO4 | 2.384 | 84.30 | 15.70 | 0.440 |
| H2SO4 | 2.152 | 76.10 | 23.90 | 0.213 |
| H2SO4+KCl | 1.902 | 67.26 | 32.74 | 0.023 |
| H2SO4+NaCl | 1.799 | 63.61 | 36.39 | -0.047 |
| H2SO4+LiCl | 1.763 | 62.34 | 37.66 | -0.071 |
| H2SO4+ZnCl2 | 1.682 | 59.48 | 40.52 | -0.123 |
| H2SO4+NaHSO4 | 1.484 | 52.48 | 47.52 | -0.247 |
| H2SO4+MgCl2 | 1.346 | 47.60 | 52.40 | -0.332 |
Table 6 H0 of aqueous solution system of sulfuric acid+salt
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| o⁃Nitroaniline | 2.828 | 100.00 | 0 | — |
| H2SO4+Na2HPO4 | 2.797 | 98.90 | 1.10 | 1.665 |
| H2SO4+NaH2PO4 | 2.646 | 93.56 | 6.44 | 0.873 |
| H2SO4+Na2SO4 | 2.384 | 84.30 | 15.70 | 0.440 |
| H2SO4 | 2.152 | 76.10 | 23.90 | 0.213 |
| H2SO4+KCl | 1.902 | 67.26 | 32.74 | 0.023 |
| H2SO4+NaCl | 1.799 | 63.61 | 36.39 | -0.047 |
| H2SO4+LiCl | 1.763 | 62.34 | 37.66 | -0.071 |
| H2SO4+ZnCl2 | 1.682 | 59.48 | 40.52 | -0.123 |
| H2SO4+NaHSO4 | 1.484 | 52.48 | 47.52 | -0.247 |
| H2SO4+MgCl2 | 1.346 | 47.60 | 52.40 | -0.332 |
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| H2SO4 | 0.250 | 32.22 | 67.78 | 0.667 |
| H2SO4+[MIM][MSA] | 0.279 | 35.94 | 64.06 | 0.739 |
| H2SO4+[MIM][TFO] | 0.254 | 32.72 | 67.28 | 0.677 |
| H2SO4+[MIM][HSO4] | 0.238 | 30.68 | 69.32 | 0.636 |
| H2SO4+[MIM][H2PO4] | 0.436 | 56.19 | 43.81 | 1.098 |
Table 7 H0 of aqueous solution systems of sulfuric acid+ionic liquids
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| H2SO4 | 0.250 | 32.22 | 67.78 | 0.667 |
| H2SO4+[MIM][MSA] | 0.279 | 35.94 | 64.06 | 0.739 |
| H2SO4+[MIM][TFO] | 0.254 | 32.72 | 67.28 | 0.677 |
| H2SO4+[MIM][HSO4] | 0.238 | 30.68 | 69.32 | 0.636 |
| H2SO4+[MIM][H2PO4] | 0.436 | 56.19 | 43.81 | 1.098 |
| [1] | Wang Z. Y., Su Z. Y., Xu Y., Qi J. G., Qi B. H., Wei X. Y., Chen X. J., Hu Y. F., Liu Z. C., Guo X., ACS Sustain. Chem. Eng., 2024, 12(37), 14087—14098 |
| [2] | Yuan K., Zhang T., Lv L., Wang Y., Zou Z. P., Tang S. W., Ind. Eng. Chem. Res., 2024, 63(28), 12440—12451 |
| [3] | Li F., Zhang T., Lv L., Tang W. X., Wang Y., Tang S. W., Chinese J. Chem. Eng., 2024, 73, 42—50 |
| [4] | Wang Z. Y., Wei X. Y., Qi B. H., Chen X. J., Pi J. J., Yang Y., Zhang J. H., Wang N. N., Jiang S. Q., Huang Q. Z., Gao N., Hu Y. F., Liu Z. C., Guo X., J. Chem. Eng. Data, 2024, 69(12), 4430—4437 |
| [5] | Grützner T., Hasse H., Lang N., Siegert M., Ströfer E., Chem. Eng. Sci., 2007, 62(18—20), 5613—5620 |
| [6] | Yang W. F., Inner Mongolia Petrochemical Industry, 2021, 47(3), 32—35 |
| 杨文峰. 内蒙古石油化工, 2021, 47(3), 32—35 | |
| [7] | Sood K., Saini Y., Thakur K. K., Mater. Todays: Proc., 2023, 81, 739—744 |
| [8] | Wang D. L., Li D., Guangzhou Chemical Industry, 2022, 50(5), 81—84 |
| 王大六, 李丹. 广州化工, 2022, 50(5), 81—84 | |
| [9] | Ren C. X., Li J. S., Wang J. J., Jiang S. Q., Guo X., Qi J. G., Jiao C. Z., Wang Y. C., Hu Y. F., Liu Z. C., J. Chem. Technol. Biot., 2022, 97(5), 1275—1279 |
| [10] | Gao N., Yang Y., Wang Z. Y., Guo X., Jiang S. Q., Li J. S., Hu Y. F., Liu Z. C., Xu C. M., Chem. Rev., 2024, 124(1), 27—123 |
| [11] | Zhou T., Gui C. M., Sun L. G., Hu Y. X., Lyu H., Wang Z. H., Song Z., Yu G. Q., Chem. Rev., 2023, 123(21), 12170—12253 |
| [12] | Tang X., Lv S. Y., Jiang K., Zhou G. H., Liu X. M., J. Power Sources, 2022, 542, 231792 |
| [13] | Zheng D. X., Li D., Huang W. J., Wu X. H., Nie N., Renew. Sust. Energ. Rev., 2014, 37, 47—68 |
| [14] | Ma C. Y., Shukla S. K., Samilkannu R., Mikkola J. P., Ji X. Y., ACS Sustain. Chem. Eng., 2020, 8(1), 415—426 |
| [15] | Liu C. Z., Wang F., Stiles A. R., Guo C., Appl. Energ., 2012, 92, 406—414 |
| [16] | Schneider S., Hawkins T., Rosander M., Vaghjiani G., Chambreau S., Drake G., Energy Fuels, 2008, 22(4), 2871—2872 |
| [17] | Zhou Q., Zhao Y. Y., Guo L. Y., Shi Y. F., Zheng R. R., Chem. J. Chinese Universities, 2024, 45(5), 20230488 |
| 周俏, 赵圆圆, 郭立颖, 史亚飞, 郑荣荣. 高等学校化学学报, 2024, 45(5), 20230488 | |
| [18] | Zhang S., Zhang T., Tang S. W., J. Chem. Eng. Data, 2016, 61(6), 2088—2097 |
| [19] | Ivanenko T. Y., Kondrasenko A. A., Rubaylo A. I., J. Mol. Liq., 2023, 391, 123438 |
| [20] | Long F. A., Mclntyre D., J. Am. Chem. Soc., 1954, 76(12), 3243—3247 |
| [21] | Paul M. A., J. Am. Chem. Soc., 1954, 76(12), 3236—3239 |
| [22] | Harbottle G., J. Am. Chem. Soc., 1951, 73(8), 4024—4025 |
| [23] | Ling S., Experiment and Theory Study on Physicochemical Properties of Pyrrolidonium Ionic Liquids and Its Multicomponent Aqueous Solutions, China University of Petroleum (Beijing), Beijing, 2012 |
| 凌山. 吡咯烷酮类离子液体及其多元水溶液物性的实验和理论研究, 北京: 中国石油大学(北京), 2012 | |
| [24] | Wang Z. X., Experiment and Theory Study on Physicochemical Properties of Novel Ionic Liquids and Synthesis of Trioxane by Formaldehyde with These Ionic Liquid Catalysts, China University of Petroleum (Beijing), Beijing, 2013 |
| 王智鑫. 新型离子液体的物性及催化甲醛合成三聚甲醛反应的理论和实验研究, 北京: 中国石油大学(北京), 2013 | |
| [25] | Huang H. Z., Experiment and Theory Study on Physicochemical Properties of Novel Ionic Liquids and Synthesis of Trioxane by Formaldehyde with These Ionic Liquid Catalyst Systems, China University of Petroleum (Beijing), Beijing, 2015 |
| 黄和志. 新型离子液体的物性及催化合成三聚甲醛反应的理论和实验研究, 北京: 中国石油大学(北京), 2015 | |
| [26] | Guo X., Wang Z. Y., Yang Y., Zhang J. H., Liu Y. D., Mu Z. Y., Jiang S. Q., Ren C. X., Lv D., Hu Y. F., Liu Z. C., Green Chem. Eng., 2024, 5(1), 108—118 |
| [27] | Zhou F., Zhang Y., Zhang T., Liang B., Tang S. W., Natural Gas Chemical Industry, 2013, 38(6), 87—91 |
| 周飞, 张圆, 张涛, 梁斌, 唐盛伟. 天然气化工(C1化学与化工), 2013, 38(6), 87—91 | |
| [28] | Cindioglu A., Ibrahim A. S. I., Sonmez O., Fuel, 2025, 382, 133791 |
| [29] | Guo H., Li H. N., Cao X. Y., Wang Z. Y., Zhang Q., Zhang G. B., Green Process. Synth., 2020, 9(1), 554—558 |
| [30] | Han B. Y., Jiang J. H., Zhang W. D., Yin F., Liu S. Q., Zhao X. L., Liu J., Wang C. M., Yang H., Energ. Source. Part A, 2019, 41(20), 2448—2459 |
| [31] | Fang J. H., Wang L., Chen Z. Y., Wang S., Yuan L., Saeed A., Hussain I., Zhao J. W., Liu R. X., Miao Q. Q., ACS Appl. Mater. Interfaces, 2024, 16(18), 23443—23451 |
| [32] | Gu Y. L., Zhang J., Duan Z. Y., Deng Y. Q., Adv. Synth. Catal., 2005, 347(4), 512—516 |
| [33] | Li Z., Zhao Y. W., Han F., Yang L., Song H. Y., Chen J., Xia C. G., Scientia Sinica Chimica, 2012, 42(4), 502—524 |
| 李臻, 赵应伟, 韩峰, 杨磊, 宋河远, 陈静, 夏春谷. 中国科学: 化学, 2012, 42(4), 502—524 | |
| [34] | Qi J. G., Hu Y. F., Ma W. T., Wang H. Y., Jiang S. Q., Yin L. Y., Zhang X. M., Yang Z. Y., Wang Y. C., Chem. Eng. J., 2018, 331, 311—316 |
| [35] | Yin L. Y., Hu Y. F., Zhang X. M., Qi J. G., Ma W. T., RSC Adv., 2015, 5, 37697—37702 |
| [1] | REN Shufang, GUO Tong, WANG Zihan, LIU Yahui, CHEN Yu, ZENG Junling. Construction of Molecular Imprinted Electrochemical Sensor Based on 2D Ti3C2T x Nanosheet/Conductive Kochen Black Composite Polymethacrylic Acid and the Detection of Dopamine [J]. Chem. J. Chinese Universities, 2025, 46(7): 20250040. |
| [2] | LI Dan, HU Honghui, HOU Hongshuai, ZHANG Sheng, LIU Lijie, JING Mingjun, WU Tianjing. Sodium Storage Performance of Mixed-phase Sodium Titanate Tuned by Carbon Dots [J]. Chem. J. Chinese Universities, 2025, 46(6): 20240356. |
| [3] | LIU Jinkun, RAN Zhun, LIU Qingqing, LIU Yingliang, ZHUANG Jianle, HU Chaofan. Preparation of Carbon Dot-based Multicolor Room-temperature Phosphorescent Materials via Precursor Structure Regulation Strategies [J]. Chem. J. Chinese Universities, 2025, 46(6): 20240412. |
| [4] | LUO Kui, LIN Jiaxi, LI Jianping. Development of a Glycosyl-imprinted Sensor and Rapid Detection of PD-L1 Positive Exosomes in Breast Cancer [J]. Chem. J. Chinese Universities, 2025, 46(5): 20240524. |
| [5] | XU Xingyu, XIE Xiaoming, QIU Ping. Ring-opening Mechanism of 2-Phenyl-3-amineazetidine to Form Thiazole or Oxazole [J]. Chem. J. Chinese Universities, 2025, 46(5): 20240547. |
| [6] | HONG Yang, LI Dandan, ZHANG Jingshun, ZHANG Ziwang, GAO Guohua. Porous Poly(ionic liquid)s-catalyzed Carbon Dioxide-promoted Hydration of Ethylene Oxide [J]. Chem. J. Chinese Universities, 2025, 46(5): 20240570. |
| [7] | KANG Sha, ZHANG Ke, WEI Yajing, WANG Chuanyi. In⁃situ Construction of N-defective g-C3N5/CdS/Ti3C2 Schottky Junction for High-efficiency Photocatalytic NO Removal [J]. Chem. J. Chinese Universities, 2025, 46(4): 20240488. |
| [8] | ZHENG Na, NIE Lijun, GAO Yuhang, XUE Kunkun, HAN Xiaobei, MA Yueyu, REN Lirong, SU Wangchao, SHI Jianhui. H3PO4 Protonation-modified g-C3N4 and Its Photocatalytic H2O2 Production Properties [J]. Chem. J. Chinese Universities, 2025, 46(4): 20240485. |
| [9] | JIANG Yanli, XU Yunsong, WANG Jiankang, LI Weihao, SONG Ying, WANG Xinzhi, YAO Zhongping. Preparation of Ti3C2-MXene/CuS/PVDF Composite Photothermal Membrane and Its Solar-driven Interfacial Water Evaporation Performance [J]. Chem. J. Chinese Universities, 2025, 46(4): 20240469. |
| [10] | LIU Yixuan, HU Huimin, FAN Xiaoqiang, YU Xuehua, KONG Lian, XIAO Xia, XIE Zean, ZHAO Zhen. Preparation of Pt/Mn-silicalite-1 Catalysts and Their Catalytic Performance for Propane Dehydrogenation [J]. Chem. J. Chinese Universities, 2025, 46(3): 20240460. |
| [11] | SHEN Yuhao, TIAN Zemin, LI Wei, JI Yixuan, YAN Yingwen. Theoretical Study of the Effect of Conformational Structures on the Secondary Oxidation Reactions of cis-1,3-Dimethylcyclohexane [J]. Chem. J. Chinese Universities, 2025, 46(3): 20240458. |
| [12] | DIAO Zhenheng, LI Hao, GUO Wen, ZHENG Pengfei, WANG Bin, JI Honglun, TIAN Yajie, SUN De, LI Li. Fabrication of Core-shell Structured Monoliths and Their Catalytic Performance for Chlorobenzene Combustion [J]. Chem. J. Chinese Universities, 2025, 46(3): 20240394. |
| [13] | LIU Meng, XU Yi, YANG Fan, ZHOU Quan, REN Jing, REN Ruipeng, LYU Yongkang. Synthesis of COF-LZU1 in Acetate Buffer and Immobilized Enzyme Study [J]. Chem. J. Chinese Universities, 2025, 46(2): 20240368. |
| [14] | WANG Lanyi, WANG Shiwei, CHEN Xinyu, YU Di, ZHANG Chunlei, FAN Xiaoqiang, YU Xuehua, ZHAO Zhen. Preparation of MO δ /3DOM ZSM-5 Catalysts and Their Catalytic Performance for the Simultaneous Removal of Soot and NOx [J]. Chem. J. Chinese Universities, 2025, 46(2): 20240455. |
| [15] | LIU Xiaokang, ZHOU Yuxiu, LI Xiaoyong, LUO Wenjing, WANG Kehu, WANG Junjiao, HUANG Danfeng, HU Yulai. Synthesis of Difluoromethyl Pyrazoles by [3+2] Cycloaddition Reaction of Difluoroacetohydrazonoyl Bromides with Vinyl Sulfonefones [J]. Chem. J. Chinese Universities, 2025, 46(2): 20240302. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||