

Chem. J. Chinese Universities ›› 2021, Vol. 42 ›› Issue (12): 3709.doi: 10.7503/cjcu20210495
• Physical Chemistry • Previous Articles Next Articles
Received:2021-07-13
Online:2021-12-10
Published:2021-12-08
Contact:
WANG Baoshan
E-mail:baoshan@whu.edu.cn
Supported by:CLC Number:
TrendMD:
HOU Hua, WANG Baoshan. Group Additivity Theoretical Model for the Prediction of Dielectric Strengths of the Alternative Gases to SF6[J]. Chem. J. Chinese Universities, 2021, 42(12): 3709.
| No. | Formula | Er,exp. | Er(GA) | Er(QSAR) | No. | Formula | Er,exp. | Er(GA) | Er(QSAR) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SF6 | 1 | 0.97 | 0.87 | 34 | SO2F2 | 0.73 | 0.78 | 0.82 |
| 2 | SF5CF3 | 1.53 | 1.56 | 1.29 | 35 | CF3SO2F | 1.43 | 1.37 | 1.32 |
| 3 | SeF6 | 1.21 | 1.21 | 0.81 | 36 | CF3CN | 1.5 | 1.65 | 1.68 |
| 4 | CF4 | 0.43 | 0.41 | 0.69 | 37 | C2F5CN | 1.98 | 1.98 | 1.92 |
| 5 | CCl4 | 2.36 | 2.35 | 1.54 | 38 | n?C3F7CN | 2.5 | 2.31 | 2.15 |
| 6 | CF3Cl | 0.6 | 0.64 | 0.87 | 39 | i?C3F7CN | 2.2 | 2.06 | 2.02 |
| 7 | CF3I | 1.21 | 1.23 | 0.84 | 40 | CF3OCF(CF3)CN | 1.94 | 2.10 | 2.28 |
| 8 | CFCl3 | 1.84 | 2.12 | 1.41 | 41 | (CF3)2O | 0.84 | 0.84 | 1.24 |
| 9 | CF2Cl2 | 1.06 | 1.04 | 1.18 | 42 | CF3OC2F5 | 1.24 | 1.18 | 1.63 |
| 10 | CF2ClBr | 1.32 | 1.23 | 1.16 | 43 | (C2F5)2O | 1.51 | 1.51 | 1.98 |
| 11 | CF3Br | 0.76 | 0.76 | 0.88 | 44 | c?C4F8O | 1.38 | 1.38 | 1.57 |
| 12 | C2F6 | 0.8 | 0.77 | 1.07 | 45 | (CF3)2S | 1.5 | 1.50 | 1.32 |
| 13 | C3F8 | 0.98 | 1.10 | 1.39 | 46 | C2F5C(=O)F | 1.59 | 1.65 | 1.98 |
| 14 | C4F10 | 1.32 | 1.42 | 1.69 | 47 | i?C3F7C(=O)CF3 | 2.1 | 2.10 | 2.34 |
| 15 | C5F12 | 1.75 | 1.75 | 1.98 | 48 | i?C3F7C(=O)C2F5 | 2.7 | 2.43 | 2.67 |
| 16 | C6F14 | 2.26 | 2.08 | 2.31 | 49 | CF3NO2 | 1.34 | 1.34 | 1.18 |
| 17 | CF3CF2Cl | 1.15 | 1.24 | 1.30 | 50 | SF3≡N | 1.37 | 1.37 | 1.36 |
| 18 | CF2ClCF2Cl | 1.71 | 1.71 | 1.53 | 51 | CF3N=SF2 | 2.41 | 2.41 | 1.25 |
| 19 | CF2ClCFCl2 | 2.37 | 2.19 | 1.80 | 52 | CH3Cl | 0.32 | 0.33 | 0.34 |
| 20 | CF3CCl3 | 2.47 | 2.48 | 1.77 | 53 | CH3Br | 0.45 | 0.45 | 0.48 |
| 21 | c?C4F8 | 1.28 | 1.28 | 1.49 | 54 | CH3I | 1.12 | 0.92 | 0.59 |
| 22 | c?C6F12[c?C4F6(CF3)2] | 2.35 | 2.30 | 2.09 | 55 | CH2Cl2 | 0.64 | 0.73 | 0.85 |
| 23 | c?C7F14[c?C6F11CF3] | 2.24 | 2.43 | 2.04 | 56 | CHCl3 | 1.77 | 1.76 | 1.17 |
| 24 | c?C4F6 | 1.6 | 1.53 | 1.07 | 57 | CHF3 | 0.27 | 0.06 | 0.04 |
| 25 | c?C5F8 | 2.1 | 1.85 | 1.52 | 58 | CHF2Cl | 0.44 | 0.53 | 0.63 |
| 26 | c?C6F10 | 2.05 | 2.17 | 1.94 | 59 | CHFCl2 | 0.92 | 1.00 | 1.01 |
| 27 | CF2=CFCl | 0.69 | 0.69 | 1.12 | 60 | CH2F2 | 0.27 | 0.35 | 0.21 |
| 28 | CF2=CFCF3 | 1.01 | 1.16 | 1.13 | 61 | CH2FCl | 0.39 | 0.39 | 0.78 |
| 29 | CF3CF=CFCF3 | 1.66 | 1.66 | 1.61 | 62 | CH3CH2Cl | 0.85 | 0.85 | 0.89 |
| 30 | CF2=CF—CF=CF2 | 1.4 | 1.54 | 1.50 | 63 | CHF=CHCF3 | 0.4 | 0.35 | 0.85 |
| 31 | CF3C≡CCF3 | 2.19 | 2.19 | 1.52 | 64 | CH3CF3 | 0.41 | 0.46 | 0.46 |
| 32 | C6F6 | 1.4 | 1.40 | 1.40 | 65 | CH2=CHCF3 | 0.8 | 0.80 | 1.05 |
| 33 | SOF2 | 1.42 | 1.42 | 0.99 |
| No. | Formula | Er,exp. | Er(GA) | Er(QSAR) | No. | Formula | Er,exp. | Er(GA) | Er(QSAR) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SF6 | 1 | 0.97 | 0.87 | 34 | SO2F2 | 0.73 | 0.78 | 0.82 |
| 2 | SF5CF3 | 1.53 | 1.56 | 1.29 | 35 | CF3SO2F | 1.43 | 1.37 | 1.32 |
| 3 | SeF6 | 1.21 | 1.21 | 0.81 | 36 | CF3CN | 1.5 | 1.65 | 1.68 |
| 4 | CF4 | 0.43 | 0.41 | 0.69 | 37 | C2F5CN | 1.98 | 1.98 | 1.92 |
| 5 | CCl4 | 2.36 | 2.35 | 1.54 | 38 | n?C3F7CN | 2.5 | 2.31 | 2.15 |
| 6 | CF3Cl | 0.6 | 0.64 | 0.87 | 39 | i?C3F7CN | 2.2 | 2.06 | 2.02 |
| 7 | CF3I | 1.21 | 1.23 | 0.84 | 40 | CF3OCF(CF3)CN | 1.94 | 2.10 | 2.28 |
| 8 | CFCl3 | 1.84 | 2.12 | 1.41 | 41 | (CF3)2O | 0.84 | 0.84 | 1.24 |
| 9 | CF2Cl2 | 1.06 | 1.04 | 1.18 | 42 | CF3OC2F5 | 1.24 | 1.18 | 1.63 |
| 10 | CF2ClBr | 1.32 | 1.23 | 1.16 | 43 | (C2F5)2O | 1.51 | 1.51 | 1.98 |
| 11 | CF3Br | 0.76 | 0.76 | 0.88 | 44 | c?C4F8O | 1.38 | 1.38 | 1.57 |
| 12 | C2F6 | 0.8 | 0.77 | 1.07 | 45 | (CF3)2S | 1.5 | 1.50 | 1.32 |
| 13 | C3F8 | 0.98 | 1.10 | 1.39 | 46 | C2F5C(=O)F | 1.59 | 1.65 | 1.98 |
| 14 | C4F10 | 1.32 | 1.42 | 1.69 | 47 | i?C3F7C(=O)CF3 | 2.1 | 2.10 | 2.34 |
| 15 | C5F12 | 1.75 | 1.75 | 1.98 | 48 | i?C3F7C(=O)C2F5 | 2.7 | 2.43 | 2.67 |
| 16 | C6F14 | 2.26 | 2.08 | 2.31 | 49 | CF3NO2 | 1.34 | 1.34 | 1.18 |
| 17 | CF3CF2Cl | 1.15 | 1.24 | 1.30 | 50 | SF3≡N | 1.37 | 1.37 | 1.36 |
| 18 | CF2ClCF2Cl | 1.71 | 1.71 | 1.53 | 51 | CF3N=SF2 | 2.41 | 2.41 | 1.25 |
| 19 | CF2ClCFCl2 | 2.37 | 2.19 | 1.80 | 52 | CH3Cl | 0.32 | 0.33 | 0.34 |
| 20 | CF3CCl3 | 2.47 | 2.48 | 1.77 | 53 | CH3Br | 0.45 | 0.45 | 0.48 |
| 21 | c?C4F8 | 1.28 | 1.28 | 1.49 | 54 | CH3I | 1.12 | 0.92 | 0.59 |
| 22 | c?C6F12[c?C4F6(CF3)2] | 2.35 | 2.30 | 2.09 | 55 | CH2Cl2 | 0.64 | 0.73 | 0.85 |
| 23 | c?C7F14[c?C6F11CF3] | 2.24 | 2.43 | 2.04 | 56 | CHCl3 | 1.77 | 1.76 | 1.17 |
| 24 | c?C4F6 | 1.6 | 1.53 | 1.07 | 57 | CHF3 | 0.27 | 0.06 | 0.04 |
| 25 | c?C5F8 | 2.1 | 1.85 | 1.52 | 58 | CHF2Cl | 0.44 | 0.53 | 0.63 |
| 26 | c?C6F10 | 2.05 | 2.17 | 1.94 | 59 | CHFCl2 | 0.92 | 1.00 | 1.01 |
| 27 | CF2=CFCl | 0.69 | 0.69 | 1.12 | 60 | CH2F2 | 0.27 | 0.35 | 0.21 |
| 28 | CF2=CFCF3 | 1.01 | 1.16 | 1.13 | 61 | CH2FCl | 0.39 | 0.39 | 0.78 |
| 29 | CF3CF=CFCF3 | 1.66 | 1.66 | 1.61 | 62 | CH3CH2Cl | 0.85 | 0.85 | 0.89 |
| 30 | CF2=CF—CF=CF2 | 1.4 | 1.54 | 1.50 | 63 | CHF=CHCF3 | 0.4 | 0.35 | 0.85 |
| 31 | CF3C≡CCF3 | 2.19 | 2.19 | 1.52 | 64 | CH3CF3 | 0.41 | 0.46 | 0.46 |
| 32 | C6F6 | 1.4 | 1.40 | 1.40 | 65 | CH2=CHCF3 | 0.8 | 0.80 | 1.05 |
| 33 | SOF2 | 1.42 | 1.42 | 0.99 |
| No. | Functional group j | Er,j | No. | Functional group j | Er,j |
|---|---|---|---|---|---|
| 1 | CF | 0.0245 | 18 | ―C≡N | 1.2680 |
| 2 | CCl | 0.2568 | 19 | ―S≡N | 0.8833 |
| 3 | CBr | 0.3772 | 20 | >C=O | 0.9172 |
| 4 | CI | 0.8477 | 21 | >S=O | 1.0956 |
| 5 | SF | 0.1622 | 22 | >S(=O)2 | 0.4529 |
| 6 | SeF | 0.2017 | 23 | >S=N― | 1.9863 |
| 7 | >CF2, =CF2 | 0.3259 | 24 | SCF3 | 0.7500 |
| 8 | c?CF2 | 0.3200 | 25 | OCF3 | 0.4200 |
| 9 | >CCl2, =CCl2 | 0.7127 | 26 | NCF3 | 0.0992 |
| 10 | >CFCl, =CFCl | 0.3672 | 27 | ―OCF2― | 0.3700 |
| 11 | CF3 | 0.3861 | 28 | ―NO2 | 0.9539 |
| 12 | CCl3 | 2.0918 | 29 | =CH | 0.01 |
| 13 | CF2Cl | 0.8550 | 31 | =CH2 | 0.4039 |
| 14 | CFCl2 | 1.3324 | 32 | CH | -0.3296 |
| 15 | =CF―, c?CF | 0.4439 | 33 | CH2 | 0.0200 |
| 16 | Aromatic CF | 0.2333 | 34 | CH3 | 0.0722 |
| 17 | ―C≡C― | 1.4178 |
| No. | Functional group j | Er,j | No. | Functional group j | Er,j |
|---|---|---|---|---|---|
| 1 | CF | 0.0245 | 18 | ―C≡N | 1.2680 |
| 2 | CCl | 0.2568 | 19 | ―S≡N | 0.8833 |
| 3 | CBr | 0.3772 | 20 | >C=O | 0.9172 |
| 4 | CI | 0.8477 | 21 | >S=O | 1.0956 |
| 5 | SF | 0.1622 | 22 | >S(=O)2 | 0.4529 |
| 6 | SeF | 0.2017 | 23 | >S=N― | 1.9863 |
| 7 | >CF2, =CF2 | 0.3259 | 24 | SCF3 | 0.7500 |
| 8 | c?CF2 | 0.3200 | 25 | OCF3 | 0.4200 |
| 9 | >CCl2, =CCl2 | 0.7127 | 26 | NCF3 | 0.0992 |
| 10 | >CFCl, =CFCl | 0.3672 | 27 | ―OCF2― | 0.3700 |
| 11 | CF3 | 0.3861 | 28 | ―NO2 | 0.9539 |
| 12 | CCl3 | 2.0918 | 29 | =CH | 0.01 |
| 13 | CF2Cl | 0.8550 | 31 | =CH2 | 0.4039 |
| 14 | CFCl2 | 1.3324 | 32 | CH | -0.3296 |
| 15 | =CF―, c?CF | 0.4439 | 33 | CH2 | 0.0200 |
| 16 | Aromatic CF | 0.2333 | 34 | CH3 | 0.0722 |
| 17 | ―C≡C― | 1.4178 |
| Species | Molecular structure | Experimental value | GA procedure |
|---|---|---|---|
| CF3SO2F | 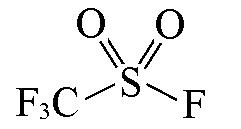 | 1.43 | {1×S(=O)2+1×SCF3+1×SF}= 0.4529+0.7500+0.1622=1.37 |
| c?C6F12 | 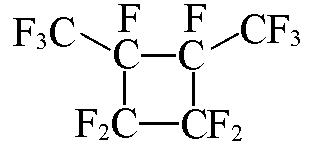 | 2.35 | {2×c?CF2+2×c?CF+2×CF3}= 2×0.3200+2×0.4439+2×0.3861=2.30 |
| c?C4F8O | 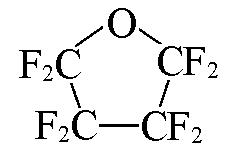 | 1.38 | {2×c?CF2+2×OCF2}= 2×0.3200+2×0.37=1.38 |
| CF3N=SF2 | 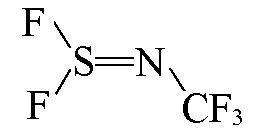 | 2.41 | {1×S=N+2×SF+1×NCF3}= 1×1.9863+2×0.1622+1×0.0992=2.41 |
| CHF=CHCF3 | 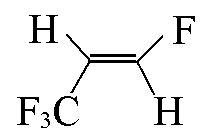 | 0.85 | {1×CF3+2×=CH+1×=CF}= 0.3861+2×0.01+1×0.4439=0.85 |
| i?C3F7CN | 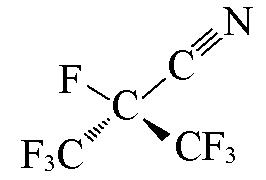 | 2.20 | {1×C≡N+1×CF+2×CF3}= 1×1.268+1×0.0245+2×0.3861=2.06 |
| i?C3F7C(=O)CF3 | 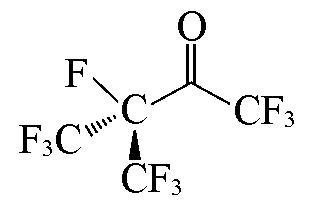 | 2.10 | {1×C=O+3×CF3+1×CF}= 1×0.9172+3×0.3861+1×0.0245=2.10 |
| Species | Molecular structure | Experimental value | GA procedure |
|---|---|---|---|
| CF3SO2F |  | 1.43 | {1×S(=O)2+1×SCF3+1×SF}= 0.4529+0.7500+0.1622=1.37 |
| c?C6F12 |  | 2.35 | {2×c?CF2+2×c?CF+2×CF3}= 2×0.3200+2×0.4439+2×0.3861=2.30 |
| c?C4F8O |  | 1.38 | {2×c?CF2+2×OCF2}= 2×0.3200+2×0.37=1.38 |
| CF3N=SF2 | 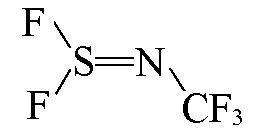 | 2.41 | {1×S=N+2×SF+1×NCF3}= 1×1.9863+2×0.1622+1×0.0992=2.41 |
| CHF=CHCF3 |  | 0.85 | {1×CF3+2×=CH+1×=CF}= 0.3861+2×0.01+1×0.4439=0.85 |
| i?C3F7CN |  | 2.20 | {1×C≡N+1×CF+2×CF3}= 1×1.268+1×0.0245+2×0.3861=2.06 |
| i?C3F7C(=O)CF3 |  | 2.10 | {1×C=O+3×CF3+1×CF}= 1×0.9172+3×0.3861+1×0.0245=2.10 |
| 1 | Ko M. K., Sze N. D., Wang W. C., J. Geophys. Res., 1993, 98(6), 10499—10507 |
| 2 | Ray E. A., Moore F. L., Elkins J. W., J. Geophys. Res., 2016, 122(7), 4626—4638 |
| 3 | Fang X. K., Hu X., Greet J. M., Environ. Sci. Tech., 2013, 47(6), 3848—3855 |
| 4 | Okubo H., Beroual A., IEEE Electr. Insul. Mag., 2011, 27(2), 34—42 |
| 5 | Beroual A., Haddad A., Energies, 2017, 10(8), 1216 |
| 6 | Kieffel Y., Irwin T., Ponchon P., IEEE Power Energy Mag., 2016, 14(2), 32—39 |
| 7 | Wang B. S., Yu X. J., Hou H., Trans. China Electrotech. Soc., 2020, 35(1), 21—33(王宝山, 余小娟, 侯华. 电工技术学报, 2020, 35(1), 21—33) |
| 8 | Rabie M., Franck C. M., Environ. Sci. Tech., 2018, 52(2), 369—380 |
| 9 | Rabie M., Dahl D. A., Donald S. M. A., IEEE Trans. Dielectr. Electr. Insul., 2013, 20(3), 856—863 |
| 10 | Yu X., Hou H., Wang B., J. Comput. Chem., 2017, 38(10), 721—729 |
| 11 | Hou H., Yu X. J., Zhou W. J., Luo Y. B., Wang B. S., Chem. J. Chinese Universities, 2018, 39(11), 2477—2484(侯华, 余小娟, 周文俊, 罗运柏, 王宝山. 高等学校化学学报, 2018, 39(11), 2477—2484) |
| 12 | Yu X., Hou H., Wang B., IEEE Electr. Insul. Conf., 2020, 38, 124—127 |
| 13 | Yu X., Hou H., Wang B., J. Phys. Chem. A, 2018, 122(13), 3462—3469 |
| 14 | Wang Y., Gao Z., Wang B., Indust. Engin. Chem. Res., 2019, 58(48), 21913—21920 |
| 15 | Deng J., Peng M., Gao Z., RSC Adv., 2020, 10(2), 2740—2746 |
| 16 | Brand K. P., IEEE Trans. Electr. Insul., 1982, 17(5), 451—456 |
| 17 | Wooton R. E., Kegelman M. R., Electric Power Research Institute(EPRI) EL⁃2620, 1982, 4—37 |
| 18 | Devins J. C., IEEE Trans. Electr. Insul., 1980, 15(2), 81—86 |
| 19 | McCormick N. R., Craggs J. D., British J. Appl. Phys., 1954, 5(5), 171—173 |
| 20 | Camilli G., Plump R. E., Trans. Am. Inst. Elect. Engin., Part I: Comm. Electr., 1953, 72(2), 93—102 |
| 21 | Malik N. H., Qureshi A. H., IEEE Trans. Electr. Insul., 1979, 14(1), 1—13 |
| 22 | Widger P., Haddad A., Griffiths H., IEEE Trans. Dielectr. Electr. Insul., 2016, 23(1), 14—21 |
| 23 | Banks A. A., Rudge A. J., Nature, 1953, 171(4348), 390—391 |
| 24 | Vijh A. K., IEEE Trans. Electr. Insul., 1982, 17(1), 84—87 |
| 25 | Joback K. G., Reid R. C., Chem. Engin. Comm., 1987, 57(2), 233—243 |
| 26 | Ammon H. L., Mitchell S., Propel. Explo. Pyrotech., 1998, 23(1), 260—265 |
| 27 | Constantinou L. R., Gani R., AIChE J., 1994, 40(10), 1697—1710 |
| 28 | Ghasemitabar H., Movagharnejad K., Fluid Phase Equilib., 2016, 411(1), 13—23 |
| 29 | Fredenslund A., Russell L., Prausnitz J. M., AIChE J., 1975, 21(6), 1086 |
| [1] | CHENG Xiankun, HOU Xue, TIAN Huan, ZHANG Menglong, WEI Hao, ZHAO Zhuo. Synthesis of Macrocyclic Thiacrown Ethers and Their Selective Extraction for Ag(Ⅰ) and Tl(Ⅰ) † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1881. |
| [2] | HOU Hua, YU Xiaojuan, ZHOU Wenjun, LUO Yunbai, WANG Baoshan. Theoretical Investigations on the Structure-activity Relationship to the Dielectric Strength of the Insulation Gases† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2477. |
| [3] | HU Song-Qing*, MI Si-Qi, JIA Xiao-Lin, GUO Ai-Ling, CHEN Sheng-Hui, ZHANG Jun, LIU Xin-Yong. 3D-QSAR Study and Molecular Design of Benzimidazole Derivatives as Corrosion Inhibitors [J]. Chem. J. Chinese Universities, 2011, 32(10): 2402. |
| [4] | ZHAO Jian-Wei, WANG Nan. Electricity Characterization of Metalloprotein at Molecular Level by Conducting Atomic Force Microscopy [J]. Chem. J. Chinese Universities, 2005, 26(4): 751. |
| [5] | TANG Qing-Hu, BAI Tong-Chun, LU Yan, ZHAO Pei-Zhen, LU Jin-Suo . Interaction Parameters of the Excess Gibbs Free Energy Between Saccharides and Non-electrolytes in Formamide [J]. Chem. J. Chinese Universities, 2001, 22(2): 240. |
| [6] | FU Wei, FENG Ji-Kang, REN Ai-Min, CUI Meng, JIN Hong-Wei, WANG Jiang-Hong, SHEN Yu-Quan. Theoretical Studies of Molecules with Nonlinear Optical Third-order Susceptibilities on Pull-push Multi-cycle Electro-optical Polymer Intermediates Including Thiophene Ring [J]. Chem. J. Chinese Universities, 2000, 21(5): 771. |
| [7] | FU Wei, FENG Ji-Kang, REN Ai-Min, SUN Xiu-Yun, JIN Hong-Wei, WANG Jiang-Hong, SHEN Yu-Quan . Theoretical Studies on Second-order Nonlinear Optical Properties of a Series of Novel Push pull Polycyclic Conjugated Molecules [J]. Chem. J. Chinese Universities, 2000, 21(4): 616. |
| [8] | FU Wei, FENG Ji-Kang, REN Ai-Min, SUN Chia-Cung. Theoretical Study on Design Molecules with First Hyperpolarizabilities on a Serials of Babituric Acid and Its Derivatives [J]. Chem. J. Chinese Universities, 2000, 21(12): 1892. |
| [9] | XIE Gui-Yang, KONG Ya-Li, WANG Jun-Mei, XU Xiao-Jie, JIN Sheng . Studies on Crystal Structure, Conformation Analysis of a New Designed Hapten Containing Sulfur [J]. Chem. J. Chinese Universities, 1999, 20(6): 890. |
| [10] | YANG Guang-Fu, LIU Hua-Yin, LU Rong-Jian, YANG Hua-Zheng. Design,Synthesis and Biological Activity of Novel Hebicides Targeted ALS(Ⅵ)——The Electronic Structure of Herbicidal Pyrimidinyl(thio)ether Compounds and the Molecular Design of Novel Herbicides [J]. Chem. J. Chinese Universities, 1998, 19(2): 222. |
| [11] | FENG Ji-Kang, WANG Hai-Chuan, XIAO Chang-Yong, SUN Chia-Chong. A Theoretical Study on the Nonlinear Optical Second-order Properties of Azobenzene Series Molecules [J]. Chem. J. Chinese Universities, 1996, 17(4): 596. |
| [12] | FENG Ji-Kang, YU Hong-Shi, LI Zhi-Ru. A Study of Molecular Design on the Second-Order Nonlinear Optical Properties of Dye-Type Organic Molecules [J]. Chem. J. Chinese Universities, 1993, 14(6): 821. |
| [13] | ZHANG Ran, WANG Yu-Hong, ZHU Zhi-Hua, GUO Li-Li, XU Jian-Ping, WANG Zong-Mu, LI Wei. Solid Phase Synthesis and Biological Activities of α-h-ANF and Its Analogs [J]. Chem. J. Chinese Universities, 1992, 13(10): 1247. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
