

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (3): 505.doi: 10.7503/cjcu20190588
• Physical Chemistry • Previous Articles Next Articles
YANG Jin1,CAO Yan1,ZHANG Naidong1,2,*
Received:2019-11-13
Online:2020-02-26
Published:2019-12-31
Contact:
Naidong ZHANG
Supported by:CLC Number:
TrendMD:
YANG Jin,CAO Yan,ZHANG Naidong. Co-sensitization in the Visible Light/H2O2 System †[J]. Chem. J. Chinese Universities, 2020, 41(3): 505.
| Colored substance | A | |
|---|---|---|
| Before illumination | After illumination for 2 h | |
| Phenol red | 0.443 | 0.442 |
| Methyl orange | 1.351 | 1.353 |
| AzureⅠ | 0.512 | 0.510 |
| Methyl orange+phenol red | 1.013 | 1.016 |
| AzureⅠ+methyl orange+phenol red | 0.536 | 0.535 |
| Direct fast brown M | 0.467 | 0.469 |
| Dianil brown 3GN | 0.375 | 0.374 |
| Colored substance | A | |
|---|---|---|
| Before illumination | After illumination for 2 h | |
| Phenol red | 0.443 | 0.442 |
| Methyl orange | 1.351 | 1.353 |
| AzureⅠ | 0.512 | 0.510 |
| Methyl orange+phenol red | 1.013 | 1.016 |
| AzureⅠ+methyl orange+phenol red | 0.536 | 0.535 |
| Direct fast brown M | 0.467 | 0.469 |
| Dianil brown 3GN | 0.375 | 0.374 |
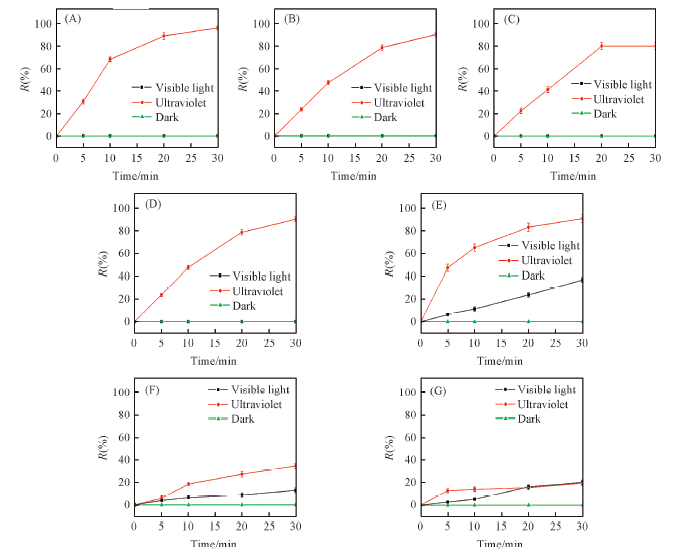
Fig.3 Chroma removal rate of different colored substance in H2O2 system under different light sources (A) Phenol red; (B) methyl orange; (C) azureⅠ; (D) methyl orange+phenol red; (E) azureⅠ+methyl orange+phenol red; (F) direct fast brown M; (G) dianil brown 3GN.
| Colored substance | ΔA510 nm | ||
|---|---|---|---|
| Ultraviolet | Visible light | Dark | |
| Phenol red(yellow) | 0.077 | 0.141 | 0 |
| Methyl orange(red) | 0.109 | 0.152 | 0 |
| AzureⅠ(blue) | 0.021 | 0.043 | 0 |
| Methyl orange+phenol red(orange) | 0.068 | 0.137 | 0 |
| AzureⅠ+methyl orange+phenol red(brown) | 0.114 | 0.157 | 0 |
| Direct fast brown M(brown) | 0.064 | 0.168 | 0 |
| Dianil brown 3GN(brown) | 0.060 | 0.186 | 0 |
| Colored substance | ΔA510 nm | ||
|---|---|---|---|
| Ultraviolet | Visible light | Dark | |
| Phenol red(yellow) | 0.077 | 0.141 | 0 |
| Methyl orange(red) | 0.109 | 0.152 | 0 |
| AzureⅠ(blue) | 0.021 | 0.043 | 0 |
| Methyl orange+phenol red(orange) | 0.068 | 0.137 | 0 |
| AzureⅠ+methyl orange+phenol red(brown) | 0.114 | 0.157 | 0 |
| Direct fast brown M(brown) | 0.064 | 0.168 | 0 |
| Dianil brown 3GN(brown) | 0.060 | 0.186 | 0 |
| Colored substance | ΔA563 nm | |
|---|---|---|
| Ultraviolet | Visible light | |
| Phenol red | 0 | 0.022 |
| Methyl orange | 0 | 0.029 |
| AzureⅠ | 0 | 0.052 |
| Methyl orange+phenol red | 0 | 0.048 |
| AzureⅠ+methyl orange+phenol red | 0 | 0.043 |
| Direct fast brown M | 0 | 0.016 |
| Dianil brown 3GN | 0 | 0.024 |
| Colored substance | ΔA563 nm | |
|---|---|---|
| Ultraviolet | Visible light | |
| Phenol red | 0 | 0.022 |
| Methyl orange | 0 | 0.029 |
| AzureⅠ | 0 | 0.052 |
| Methyl orange+phenol red | 0 | 0.048 |
| AzureⅠ+methyl orange+phenol red | 0 | 0.043 |
| Direct fast brown M | 0 | 0.016 |
| Dianil brown 3GN | 0 | 0.024 |
| Colored substance | ΔA720 nm | ||
|---|---|---|---|
| Ultraviolet | Visible light | Dark | |
| Phenol red | 0 | 0.011 | 0 |
| Methyl orange | 0 | 0.006 | 0 |
| AzureⅠ | 0 | 0.009 | 0 |
| Methyl orange+phenol red | 0 | 0.006 | 0 |
| AzureⅠ+methyl orange+phenol red | 0 | 0.076 | 0 |
| Direct fast brown M | 0 | 0.060 | 0 |
| Dianil brown 3GN | 0 | 0.057 | 0 |
| Colored substance | ΔA720 nm | ||
|---|---|---|---|
| Ultraviolet | Visible light | Dark | |
| Phenol red | 0 | 0.011 | 0 |
| Methyl orange | 0 | 0.006 | 0 |
| AzureⅠ | 0 | 0.009 | 0 |
| Methyl orange+phenol red | 0 | 0.006 | 0 |
| AzureⅠ+methyl orange+phenol red | 0 | 0.076 | 0 |
| Direct fast brown M | 0 | 0.060 | 0 |
| Dianil brown 3GN | 0 | 0.057 | 0 |
| Concentration of direct fast brown M/(mmol·L-1) | ΔA720 nm | A420 nm |
|---|---|---|
| 0.003 | 0 | 0 |
| 0.005 | 0.017 | 0 |
| 0.01 | 0.024 | 0.737 |
| 0.02 | 0.029 | 0.962 |
| 0.03 | 0.043 | 0.996 |
| Concentration of direct fast brown M/(mmol·L-1) | ΔA720 nm | A420 nm |
|---|---|---|
| 0.003 | 0 | 0 |
| 0.005 | 0.017 | 0 |
| 0.01 | 0.024 | 0.737 |
| 0.02 | 0.029 | 0.962 |
| 0.03 | 0.043 | 0.996 |
| [1] | Kumara G. R. A., Kanebo S., Okuya M., Onwona-Agyeman B., Konno A., Tennakone K ., Sol. Energy Mater. Sol. Cells, 2006, 90( 9), 1220— 1226 |
| [2] | Chen X. L., Zhang X. P., Xie Z. T., Ma Y. L ., Micronanoelectronic Technology, 2019, 5( 9), 709— 714 |
| ( 陈许龙, 张小萍, 谢占婷, 马元良 . 微纳电子技术, 2019, 5( 9), 709— 714) | |
| [3] | Duvva N., Prasanthkumar S., Giribabu L ., Solar. Energy, 2019, 184, 620— 627 |
| [4] | Galliano S., Bella F., Gerbaldi C., Falco M., Barolo C ., Energy Technology, 2017, 5( 2), 300— 311 |
| [5] | Ran H. L., Fan J. J., Zhang X. L., Mao J., Shao G ., Applied Surface Science, 2018, 430, 415— 423 |
| [6] | Ehret A., Stuhl L., Spitler M T ., J. Phys. Chem. B, 2001, 105( 41), 9960— 9965 |
| [7] | Dong L. H., Zheng Z. W., Wang Y. F., Li X., Hua J. L., Hu A. G ., J. Mater. Chem. A, 2015, 3( 21), 11607— 11614 |
| [8] | Chang C. H., Chen Y. C., Hsu C. Y., Chou H. H., Lin J. T ., Org. lett., 2012, 14( 18), 4726— 4729 |
| [9] | Bandara J., Kiwi J., Pulgarin C., Peringer P., Pajonk G. M., Elaloui A., Albers P ., Environmental Science & Technology, 1996, 30( 4), 1261— 1267 |
| [10] | Wang S. J., Liu D. J., Jiang F., Wang S. W ., Journal of Guizhou University of Technology, 2004, 33( 1), 12— 14 |
| ( 汪守建, 刘德启, 江飞, 王寿武 . 贵州工业大学学报, 2004, 33( 1), 12— 14) | |
| [11] | Bors W., Lengfelder E., Saran M., Michel C., Fuchs C., Frenzel C ., Biochemical and Biophysical Research Communications, 1977, 75( 4), 973— 979 |
| [12] | Li J. X., Wen X. R., Wu X. P ., Chinese Journal of Analysis Laboratory, 2013, 32( 3), 86— 88 |
| ( 李京雄, 温欣荣, 吴秀萍 . 分析试验室, 2013, 32( 3), 86— 88) | |
| [13] | Steiner M. G., Babbs C. F. , Archives of Biochemistry and Biophysics, 1990, 278( 2), 478— 481 |
| [14] | Xu X. R., Wang W. H., Li H. B ., Prog. Biochem. Biophys., 1999, 26( 1), 67— 69 |
| ( 徐向荣, 王文华, 李华斌 . 生物化学与生物物理进展, 1999, 26( 1), 67— 69) | |
| [15] | Sharpless C. M., Blough N. V ., Environmental Science Processes & Impacts, 2014, 16( 4), 654— 671 |
| [16] | Sun L., Chen H. M., Abdulla H. A., Mopper K ., Environmental Science Processes & Impacts, 2014, 16( 4), 757— 763 |
| [17] | Lee E., Glover C. M., Rosario-Ortiz F. L. , Environmental Science and Technology, 2013, 47, 12073— 12080 |
| [18] | Thomas-Smith T. E., Blough N. V. , Environmental Science and Technology, 2001, 35( 13), 2721— 2726 |
| [19] | Tang X. J., Wang Y. X., Kang C. L., Liu H. F., Chen B. Y., Qiu S. L ., Chem. J. Chinese Universities, 2015, 36( 9), 1719— 1723 |
| ( 唐晓剑, 王依雪, 康春莉, 刘汉飞, 陈柏言, 裘式纶 . 高等学校化学学报, 2015, 36( 9), 1719— 1723) | |
| [20] | Li Y. Y., Pan Y. H., Lian L. S., Yan S. W., Song W. H., Yang X ., Water Research, 2017, 109, 266— 273 |
| [21] | Fu X. R., Gu X. G., Lu S. G., Sharma V. K., Brusseau M. L., Xue Y. F ., Chemical Engineering Journal, 2017, 309, 22— 29 |
| [22] | Bokare A. D., Choi W ., J. Hazard. Mater., 2014, 275, 121— 135 |
| [23] | Cui H., Gu X. G., Lu S. G., Fu X. R., Zhang X ., Chemical Engineering Journal, 2017, 309, 80— 88 |
| [24] | Zhang J. B., Sun X. H., Gao Q. J., Wang H. X., Liang D. X., Liu Z. M., Han G. T., Jiang W ., Chem. J. Chinese Universities, 2019, 40( 3), 425— 430 |
| ( 张巨擘, 孙晓晗, 高桥娇, 王海鑫, 梁大鑫, 刘志明, 韩光亭, 姜伟 . 高等学校化学学报, 2019, 40( 3), 425— 430) |
| [1] | ZHAO Yuhui, LI Mingle, LONG Saran, FAN Jiangli, PENG Xiaojun. Spectroscopic Characterization of Solvation Effect for a Polarity-Sensitive BDP [J]. Chem. J. Chinese Universities, 2020, 41(9): 2018. |
| [2] | LI Xianhua, ZHANG Leigang, WANG Xuexue, YU Qingbo. Fabrication and Multi-antitumor Effect of Novel Dextran-hemin Crosslinked Micelles Triggered by Photo Conditions† [J]. Chem. J. Chinese Universities, 2015, 36(5): 1004. |
| [3] | HE Lin, CHEN Chen, LI Fei, ZHU Yi-Zhou, ZHENG Jian-Yu. Influence of Configuration on Photoinduced Electron-transfer Mechanism in Sulfonyl Amidine Linked Porphyrin-fullerene Dyad [J]. Chem. J. Chinese Universities, 2012, 33(06): 1205. |
| [4] | SHAO Xiao-Na, ZHANG Xian-Fu. Self-assembly of Phthalocyanine-carbon Nanotubes Dispersed in Micelle and Light Induced Long-lived Charge Separation State [J]. Chem. J. Chinese Universities, 2012, 33(04): 806. |
| [5] | LIU Tao, ZHANG Chong-Lei, CHEN Ping, TANG Guo-Qing, LIN Lie*. Interaction Between Methylene Blue and ctDNA by Time-resolved Spectroscopy [J]. Chem. J. Chinese Universities, 2011, 32(8): 1854. |
| [6] | XIE Pu-Hui*, GUO Feng-Qi. Preparation and Properties of Polymer Solar Cells Based on Pt(Ⅱ) Containing Polyarylacetylene [J]. Chem. J. Chinese Universities, 2010, 31(9): 1855. |
| [7] | ZHAO Hai-Ying, CHEN Chen, ZHU Yi-Zhou, ZHENG Jian-Yu*. Construction, Photophysical and Electrochemical Studies on Ferrocene-phthalocyanine-fullerene Supramolecular Triads [J]. Chem. J. Chinese Universities, 2010, 31(5): 933. |
| [8] | LÜ Qing-Luan, ZHANG Miao, YUE Ning-Ning, GONG Bin, WANG Huai-You*. Production Mechanism Study of Singlet Oxygen in Copper Ion-Catalyzed Curcumin Using Fluorimetric Method and Its Determination in the Presence of Superoxide Anion Radical [J]. Chem. J. Chinese Universities, 2009, 30(3): 460. |
| [9] | ZHANG Heng-Jun, XU Hong-Bin, ZHAO Ya-Hui, YUE Shu-Mei, LIU Si-Dong, CUI Xiu-Jun, MA Jian-Fang, SU Zhong-Min . Fluorescence Spectroscopic Study on 2-(2-Pyridyl)imidazole at Different pH Values [J]. Chem. J. Chinese Universities, 2005, 26(8): 1537. |
| [10] | SUN Tao, XU Zhu-De, JIA Zhi-Shen . Effect of Functional Groups on the Activity of Water-soluble β-Alanine C60Derivatives for Superoxide Anion Radical Scavenging [J]. Chem. J. Chinese Universities, 2003, 24(7): 1231. |
| [11] | YANG Xin-Guo, SUN Jing-Zhi, WANG Mang, CHEN Hong-Zheng, HUANG Ji . The Sensitization on Photoconductivity of Bisbenzimidazole Perylene with 5,10,15,20-Tetraphenylporphyrin in Dual-layer Photoreceptor [J]. Chem. J. Chinese Universities, 2003, 24(6): 1110. |
| [12] | ZHENG Jun-Wei, ZHOU Qun, DING Hong . Photo-induced Electron Transfer Between Adsorbed Heme Proteins and Nanosized Silver Particles [J]. Chem. J. Chinese Universities, 2003, 24(3): 419. |
| [13] | SUN Jian, LIU Ke, XU Ying-Kai, CHEN Zhong-Wei, LIU Yang. Studies on Superoxide Formation in Photosystem Ⅱ with a Novel Spin Trap [J]. Chem. J. Chinese Universities, 2002, 23(5): 979. |
| [14] | TANG Bo, LIU Yang, TANG Xiao-Ling . Studies on the Catalytic Spectrophotometry Using SDS-Schiff Cu(Ⅱ) Complex as Mimetic Enzyme [J]. Chem. J. Chinese Universities, 2001, 22(6): 919. |
| [15] | DU Ming, LIANG Fang-Zhen, TANG Bo, SHEN Han-Xi . Studies on the Determination of Superoxide Anion Radical(O2-·) and Superoxide Dismudase(SOD) Activity by Flowinjection Spectrophotometry [J]. Chem. J. Chinese Universities, 1999, 20(3): 369. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||