

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (7): 1352.doi: 10.7503/cjcu20180873
• Article: Inorganic Chemistry • Previous Articles Next Articles
LIANG Zupei1, SU Feifei2, GUO Rong2, TANG Yuyao2, JIN Shuo2, YU Zihuan2, LI Jian1,*( ), MA Shulan2,*(
), MA Shulan2,*( )
)
Received:2018-12-28
Online:2019-07-10
Published:2019-07-12
Contact:
LI Jian,MA Shulan
E-mail:ljwfu@163.com;mashulan@bnu.edu.cn
Supported by:CLC Number:
TrendMD:
LIANG Zupei, SU Feifei, GUO Rong, TANG Yuyao, JIN Shuo, YU Zihuan, LI Jian, MA Shulan. Photoluminescence Property of Layered Double Hydroxide Composite with Fluorescein and Detection Towards F
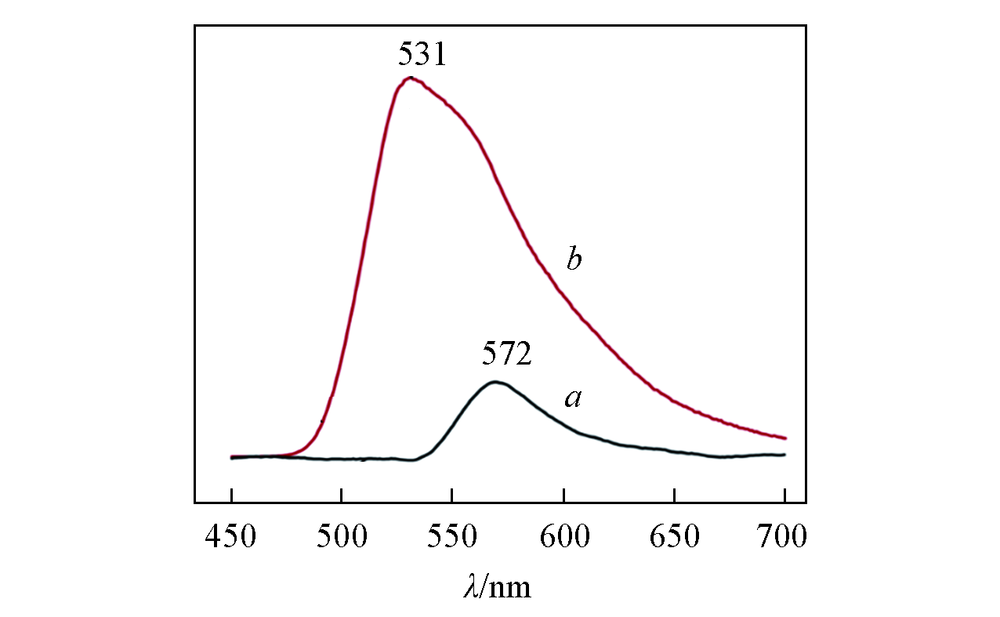
Fig.6 Emission spectra of FLN-Na(a) and delaminated FLN/OS-LDH(b) in FMa. λex=460 nm, 0.05 g FLN-Na dissolved in 20 mL FM; b. λex=460 nm, 0.05 g FLN/OS-LDH was dispersed in 20 mL FM.
| [1] | Jayaraj M. K., Vallabhan C. P. G., J. Electrochem. Soc., 1991, 138(5), 1512—1516 |
| [2] | Klemkaite K., Prosycevas I., Taraskevicius R., Khinsky A., Kareiva A., Cent. Eur. J. Chem., 2011, 9(2), 275—282 |
| [3] | Klemkaite K., Khinsky A., Kareiva A., Mater. Lett., 2011, 65(2), 388—391 |
| [4] | Wu J., Ren Z. Y., Du S. C., Kong L. J., Liu B. W.,Xi W., Zhu J. Q., Fu H. G., Nano Res., 2016, 9(3), 713—725 |
| [5] | Miyata S., Clay Clay Min., 1983, 31(4), 305—311 |
| [6] | Newman S. P., Jones W., New J. Chem., 1998, 22(2), 105—115 |
| [7] | Jaubertie C., Holgado M. J., San Roman M. S.,Rives V., Chem. Mat., 2006, 18(13), 3114—3121 |
| [8] | Klemkaite-Ramanauske K., Zilinskas A., Taraskevicius R., Khinsky A., Kareiva A., Polyhedron, 2014, 68, 340—345 |
| [9] | Kuwahara Y., Tamagawa S., Fujitani T., Yamashita H., Bull. Chem. Soc. Jpn., 2016, 89(4), 472—480 |
| [10] | Salak A. N., Tedim J., Kuznetsova A. I., Ribeiro J. L.,Vieira L. G., Zheludkevich M. L., Ferreira M. G. S., Chem. Phys., 2012, 397, 102—108 |
| [11] | Carneiro J., Caetano A. F., Kuznetsova A., Maia F., Salak A. N.,Tedim J., Scharnagl N., Zheludkevich M. L., Ferreira M. G. S., RSC Adv., 2015, 5(50), 39916—39929 |
| [12] | Li H.J., Su X. Y., Bai C. H., Xu Y. Q., Pei Z. C., Sun S. G., Sens. Actuator B-Chem., 2016, 225, 109—114 |
| [13] | Lu P., Liang S., Qiu L., Gao Y. S., Wang Q., J. Membr. Sci., 2016, 504, 196—205 |
| [14] | Serdechnova M., Salak A.N., Barbosa F. S., Vieira D. E. L., Tedim J., Zheludkevich M. L., Ferreira M. G. S., J. Solid State Chem., 2016, 233, 158—165 |
| [15] | Jiang Y., Song Y., Li Y., Tian W., Pan Z., Yang P., Li Y., Gu Q., Hu L., ACS Appl.Mater. Interfaces, 2017, 9(43), 37645—37654 |
| [16] | Hu Z. Q., Chen G. M., Adv. Mater., 2014, 26(34), 5950—5956 |
| [17] | Liu J., Chen G. M., Yang J. P., Polymer, 2008, 49(18), 3923—3927 |
| [18] | Binnemans K., Chem. Rev., 2009, 109(9), 4283—4374 |
| [19] | Gunawan P., Xu R., J. Phys. Chem. C, 2009, 113(39), 17206—17214 |
| [20] | Zhang Z., Chen G. M., Liu J. G., RSC Adv., 2014, 4(16), 7991—7997 |
| [21] | Pan Z., Jiang Y., Yang P., Wu Z., Tian W., Liu L., Song Y., Gu Q., Sun D., Hu L., ACS Nano, 2018, 12(3), 2968—2979 |
| [22] | D’Autreaux B., Tucker N. P., Dixon R., Spiro S., Nature, 2005, 437(7059), 769—772 |
| [23] | Lee J. W., Helmann J. D., Nature, 2006, 440(7082), 363—367 |
| [24] | Weinberg E.D., Eur. J. Cancer Prev., 1996, 5(1), 19—36 |
| [25] | Swaminathan S., Fonseca V.A., Alam M. G., Shah S. V., Diabetes Care, 2007, 30(7), 1926—1933 |
| [26] | Narayanaswamy N., Govindaraju T., Sens. Actuator B-Chem., 2012, 161(1), 304—310 |
| [27] | Hu Z. Q., Wang X. M., Feng Y. C., Ding L.,Li M., Lin C. S., Chem. Commun., 2011, 47(5), 1622—1624 |
| [28] | Huang J. H., Xu Y. F.,Qian X. H., Dalton Trans., 2014, 43(16), 5983—5989 |
| [29] | Kikkeri R., Traboulsi H., Humbert N., Gumienna-Kontecka E., Arad-Yellin R., Melman G., Elhabiri M., Albrecht-Gary A. M., Shanzer A., Inorg. Chem., 2007, 46(7), 2485—2497 |
| [30] | Weizman H., Ardon O., Mester B., Libman J., Dwir O., Hadar Y., Chen Y., Shanzer A., J. Am. Chem. Soc., 1996, 118(49), 12368—12375 |
| [31] | Li Z., Zhou Y., Yin K., Yu Z., Li Y., Ren J., Dyes Pigment, 2014, 105, 7—11 |
| [32] | Yang Y.T., Huo F. J., Yin C. X., Chao J. B., Zhang Y. B., Dyes Pigment, 2015, 114, 105—109 |
| [33] | Fan C. H. Huang X. M., Han L. H., Lu Z. L., Wang Z., Yi Y. P., Sens. Actuator B-Chem., 2016, 224, 592—599 |
| [34] | Li P., Zhang M., Sun X. K., Guan S. W., Zhang G., Baumgarten M.,Mullen K., Biosens. Bioelectron., 2016, 85, 785—791 |
| [35] | Li Y.F., Wang D., Liao Z., Kang Y., Ding W. H., Zheng X. J., Jin L. P., J. Mater. Chem. C, 2016, 4(19), 4211—4217 |
| [36] | Su F. F., Guo R., Yu Z. H., Li J.,Liang Z. P., Shi K. R., Ma S. L., Sun G. B., Li H. F., Dalton Trans., 2018, 47(15), 5380—5389 |
| [37] | Li J., Xie L. X., Liang Z. P., Guo R., Liu C. Y., Ma S. L., Chem. J. Chinese Universities, 2018, 39(10), 2154—2160 |
| (李建, 谢林霞, 梁足培, 国荣, 刘晨昱, 马淑兰. 高等学校化学学报, 2018, 39(10), 2154—2160) | |
| [38] | Li J., Su F. F., Guo R.,Liang Z. P., Ma S. L., Chinese J. Inorg. Chem., 2018, 34(10), 1826—1832 |
| (李建, 苏飞飞, 国荣, 梁足培, 马淑兰. 无机化学学报, 2018, 34(10), 1826—1832) | |
| [39] | Asadpour-Zeynali K., Amini R., Sens. Actuator B-Chem., 2017, 246, 961—968 |
| [40] | Ma S. L., Fan C. H., Du L., Huang G. L.,Yang X. J., Tang W. P., Makita Y., Ooi K., Chem. Mat., 2009, 21(15), 3602—3610 |
| [41] | Ma S. L., Shim Y., Islam S. M., Subrahmanyam K. S.,Wang P. L., Li H., Wang S. C., Yang X. J., Kanatzidis M. G., Chem. Mat., 2014, 26(17), 5004—5011 |
| [42] | Su F.F., Gu Q. Y., Ma S. L., Sun G. B., Yang X. J., Zhao L. D., J. Mater. Chem. C, 2015, 3(27), 7143—7152 |
| [43] | Lei S. D. Cao X. W., Feng M., Li T., Wang J., Shao M. J., Zhou Q., Wang Y. F., Journal of Light Scattering., 2007, 19(3), 290—295 |
| (雷思东, 曹学伟, 冯敏, 李涛, 王京, 邵铭杰, 周琦, 王玉芳. 光散射学报, 2007, 19(3), 290—295) | |
| [44] | Ali M., Dutta P., Pandey S., J. Phys. Chem. B, 2010, 114(46), 15042—15051 |
| [45] | Bhagi A., Pandey S., Pandey A., Pandey S., J. Phys. Chem. B, 2013, 117(17), 5230—5240 |
| [46] | Ogawa M., Kuroda K., Chem. Rev., 1995, 95(2), 399—438 |
| [47] | Dong J., Solntsev K. M., Tolbert L. M., J. Am. Chem. Soc., 2009, 131(2), 662—670 |
| [48] | Kwon M. S., Gierschner J.,Yoon S. J., Park S. Y., Adv. Mater., 2012, 24(40), 5487—5492 |
| [49] | Li S. D., Lu J., Wei M., Evans D. G.,Duan X., Adv. Funct. Mater., 2010, 20(17), 2848—2856 |
| [50] | Martwiset S., Nijpanich S., Banturngsaksiri A., Sriring M., Pandhumas T., Youngme S., J. Appl. Polym. Sci., 2013, 130(5), 3205—3211 |
| [51] | Jeong H., Lee B. I., Byeon S. H., ACS Appl. Mater. Interfaces, 2016, 8(17), 10946—10953 |
| [52] | Chen L., Wul C.L., Du P., Feng X. W., Wu P., Cai C. M., Talanta, 2017, 164, 100—109 |
| [1] | LIU Dahai, ZHANG Xueyan, FENG Yusha, DU Xianlong, XUE Longqi, DU Jianshi, ZHANG Guirong, YANG Qingbiao, LI Yaoxian. Synthesis of Novel Fluorescein-Thiospirolactams Hg2+ Fluorescent Probes and Its Application in vivo† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1412. |
| [2] |
LI Jian,XIE Linxia,LIANG Zupei,GUO Rong,LIU Chenyu,MA Shulan.
Luminescence Property and Detection Capability Towards Hg2+ of LEuH Composites with Mo |
| [3] | DU Shichao,REN Zhiyu,WU Jun,FU Honggang. Ni-Fe LDH/Reduced Graphene Oxide as Catalyst for Oxygen Evolution Reaction [J]. Chem. J. Chinese Universities, 2016, 37(8): 1415. |
| [4] | ZHANG Tao, TANG Yongjia, XU Liang, LIU Keliang. Synthesis of a New Cyanoacrylate Monomer and Its Application in Fluorescence Imaging in vivo† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1168. |
| [5] | TIAN Tao, LIU Chang, HE Song, ZENG Xianshun. Molecular Full-adder and Full-subtractor Logic Circuit Based on Fluorescein Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(2): 260. |
| [6] | SUN Mei-Yu, PANG Xiu-Jiang, MA Xiu-Ming, HOU Wan-Guo. Preparation and Particle Size Controllability of Mg-Al Layered Double Hydroxides via Coprecipitation Method Using T-type Microchannel Reactor [J]. Chem. J. Chinese Universities, 2013, 34(7): 1691. |
| [7] | MA Xiu-Ming, PANG Xiu-Jiang, QUAN Zhen-Lan, HOU Wan-Guo. Synthesis and Characterization of 10-Hydroxyl Camptothecin-Sebacate-LDH Nanohybrid [J]. Chem. J. Chinese Universities, 2013, 34(4): 913. |
| [8] | FU Hua-Kang, YE Wei-Juan, LIU Meng-Ying, HU Ji, DU Miao, ZHENG Qiang. Preparation of Layered Double Hydroxide/Carbon Nanotubes Composite and its Dispersion in Organic Solvents [J]. Chem. J. Chinese Universities, 2013, 34(3): 538. |
| [9] | CAO Tian-Chi, CHEN Guang-Ming, GUO Cun-Yue. Poly(ethylene terephthalate)/Layered Double Hydroxide Nanocomposites [J]. Chem. J. Chinese Universities, 2013, 34(10): 2239. |
| [10] | WANG Heng-Guo, LIU Da-Hai, ZHANG Chao-Qun, LI Yao-Xian, YANG Qing-Biao, DU Jian-Shi. Preparation and Characterization of Fluorescent Nanofiber Based on Fluorescein [J]. Chem. J. Chinese Universities, 2012, 33(05): 1074. |
| [11] | XU Kong-Li, CHEN Guang-Ming, SHEN Jian-Quan. Synthesis of Organically-modified Layered Double Hydroxides by Direct Decarbonation [J]. Chem. J. Chinese Universities, 2012, 33(04): 649. |
| [12] | GAN Xiao-Juan, LIU Shao-Pu, LIU Zhong-Fang, HU Xiao-Li. Fluorescence Quenching Method for the Determination of Carbazochromum with Halide Fluorescein Dyes [J]. Chem. J. Chinese Universities, 2012, 33(04): 683. |
| [13] | LIU Guo-Peng, LIU Shang-Ying, WANG Jun, SUN De-Jun*. Double Flocculation in Aqueous Dispersion of Layered Double Hydroxide Particles Induced by Sodium Polyacylate [J]. Chem. J. Chinese Universities, 2010, 31(10): 2030. |
| [14] | ZHANG Hui-Zhong, NIE Fei, Lü Jiu-Ru. Post-chemluminescence Reaction of Some Nitrogenous Organic Compounds in the N-Chlorosuccinimide-dichlorofluorescein System [J]. Chem. J. Chinese Universities, 2010, 31(1): 51. |
| [15] |
LIANG Chong-Yang1, XU Wei-Qing2, CAO Yan-Xin2, LIU Li-Xia3, ZHANG Shu-Qin1, LIU Zhi-Yi1, LI Hong-Rui1, LI Bai-Zhi1, SUN Fei1* . Dynamic Observation of Cellular Localization of Fluorescein Isothiocyanate Labeled Recombinant Ganoderma Lucidum Immunoregulatory Protein(rLz-8) in NB4 APL Cell [J]. Chem. J. Chinese Universities, 2009, 30(3): 489. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||