

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (4): 669.doi: 10.7503/cjcu20160760
• Polymer Chemistry • Previous Articles Next Articles
LIU Zhi1,2, LI Bingrui2, PAN Yanxiong2, SHI Kai2, WANG Weicai2, PENG Chao2, WANG Zhe1,*( ), JI Xiangling2,*(
), JI Xiangling2,*( )
)
Received:2016-11-01
Online:2017-04-10
Published:2017-03-22
Contact:
WANG Zhe,JI Xiangling
E-mail:wangzhe@ccut.edu.cn;xlji@ciac.ac.cn
Supported by:CLC Number:
TrendMD:
LIU Zhi, LI Bingrui, PAN Yanxiong, SHI Kai, WANG Weicai, PENG Chao, WANG Zhe, JI Xiangling. Adsorption Behavior of Hydrophilic Luffa Sponges to Heavy Metal Ions in Water System†[J]. Chem. J. Chinese Universities, 2017, 38(4): 669.
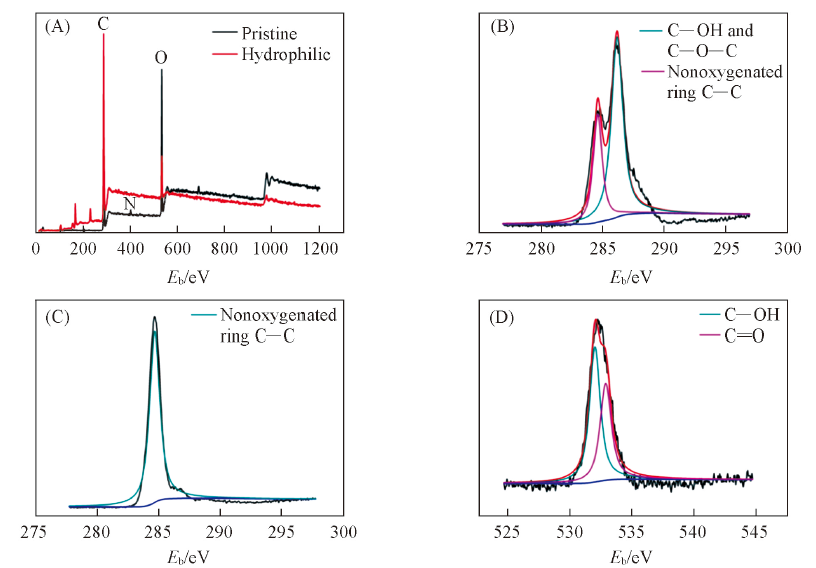
Fig.2 XPS spectra of pristine and hydrophilic[luffa-g-(PAM-co-PAANa)] luffa sponges(A), XPS spectra of C1s for pristine(B), XPS spectra of C1s(C) and O1s(D) for hydrophilic [luffa-g-(PAM-co-PAANa)] luffa sponge
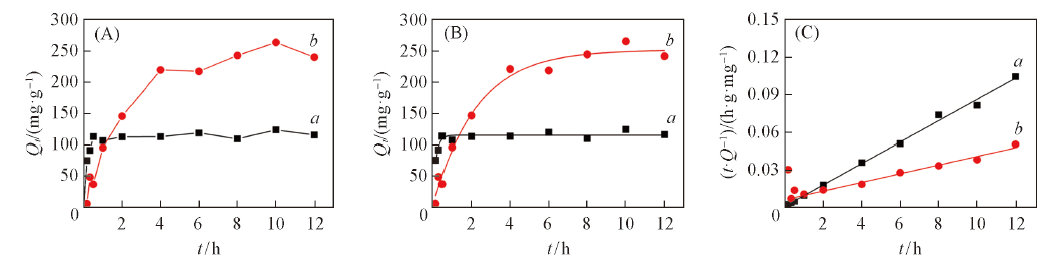
Fig.3 Adsorption kinetic curves of hydrophilic luffa sponge in Cu2+(a), Pb2+(b) solutions(20 ℃, c0=100 mg/L, pH=5.00)(A), fitted with pseudo-first-order kinetic equation(B) and pseudo-second-order kinetic equation(C)
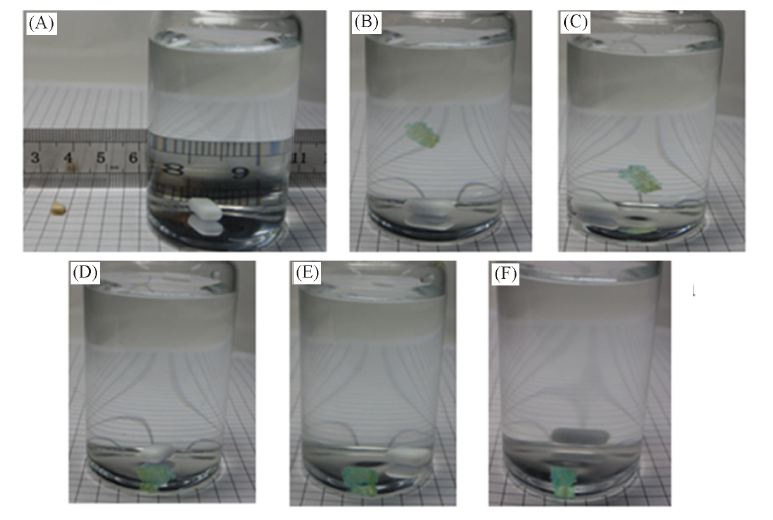
Fig.4 Adsorption procedure of hydrophilic luffa sponge for Cu2+ ions(20 ℃, c0=100 mg/L, pH=5.00) Time/min: (A) 0; (B) 10; (C) 20; (D) 30; (E) 60; (F) 120.
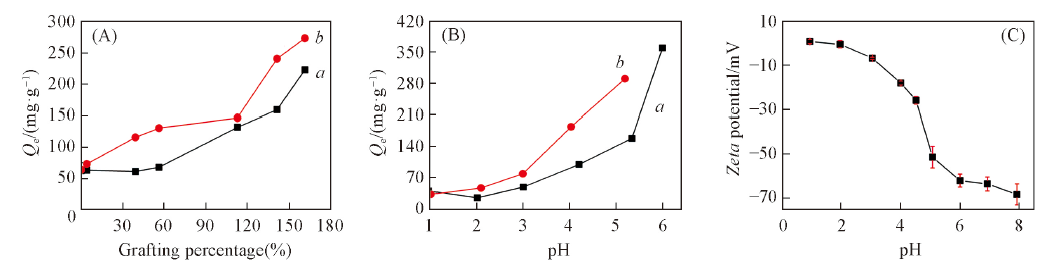
Fig.5 Saturated adsorption capacity of hydrophilic luffa sponge with different grafting percentage in Cu2+(a) and Pb2+(b) solutions(20 ℃,c0 =100 mg/L, pH=5.04)(A), pH influence on absorption capacities of hydrophilic luffa sponge for Cu2+(a) and Pb2+(b) ions at 20 ℃, c0=100 mg/L(B) and zeta potential of the sponge at different pH values(C)
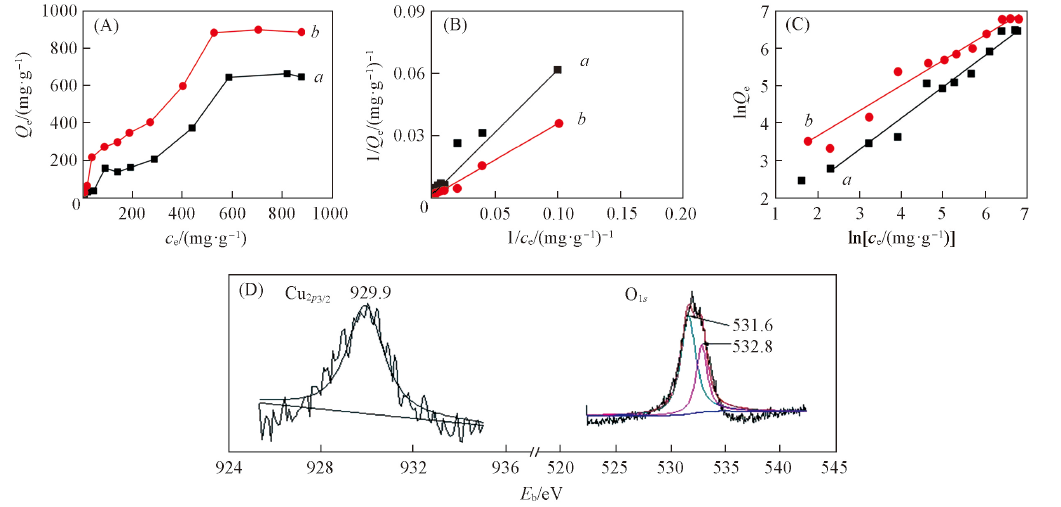
Fig.6 Adsorption capacities of hydrophilic luffa sponge in Cu2+(a), Pb2+(b) solutions with different concentrations(20 oC, pH=5.00)(A), fitted curves using Langmuir(B) and Freundlich isotherm models(C) and XPS spectra of hydrophilic luffa sponge adsorbed Cu2+ ion(D)
| Adsorbent | Absorbate | Adsorption capacity/ (mmol·g-1 or mg·g-1) | Time to reach, qe /min | pH | Reference |
|---|---|---|---|---|---|
| PVF-g-GAA | Cu2+, Pb2+, Cd2+ | 3.3—4.0 | 10 | 5.5 | [27] |
| Fe3O4@PMAA composite microspheres | Cu2+, Pb2+, Cr3+, Cd2+ | 1.0-3.5 | 5.0 | [28] | |
| Watermelon based biosorbent | Cu2+ | 31.25 | 20 | 5.0 | [29] |
| Graphene oxide-chitosan composite hydrogels | Cu2+ | 2.0 | 10 | 5.0 | [30] |
| Cassava peels | Cu2+, Pb2+ | 8.0, 5.8 | 20, 120 | 5.0 | [31] |
| Nanocrystallinecellulose | Cu2+ | 0.3—0.5 | 120 | 4.0±0.2 | [32] |
| Xylan-rich hemicelluloses-based hydrogel | Pb2+, Cd2+ | 4.1 | 60 | 3.5—6.5 | [33] |
| Hydrophilic luffa sponge | Cu2+, Pb2+ | 647, 887 | 30, 240 | 5.0 | This work |
Table 1 Adsorption performance of hydrophilic luffa sponge for heavy metal ions and comparison with values reported in the literature
| Adsorbent | Absorbate | Adsorption capacity/ (mmol·g-1 or mg·g-1) | Time to reach, qe /min | pH | Reference |
|---|---|---|---|---|---|
| PVF-g-GAA | Cu2+, Pb2+, Cd2+ | 3.3—4.0 | 10 | 5.5 | [27] |
| Fe3O4@PMAA composite microspheres | Cu2+, Pb2+, Cr3+, Cd2+ | 1.0-3.5 | 5.0 | [28] | |
| Watermelon based biosorbent | Cu2+ | 31.25 | 20 | 5.0 | [29] |
| Graphene oxide-chitosan composite hydrogels | Cu2+ | 2.0 | 10 | 5.0 | [30] |
| Cassava peels | Cu2+, Pb2+ | 8.0, 5.8 | 20, 120 | 5.0 | [31] |
| Nanocrystallinecellulose | Cu2+ | 0.3—0.5 | 120 | 4.0±0.2 | [32] |
| Xylan-rich hemicelluloses-based hydrogel | Pb2+, Cd2+ | 4.1 | 60 | 3.5—6.5 | [33] |
| Hydrophilic luffa sponge | Cu2+, Pb2+ | 647, 887 | 30, 240 | 5.0 | This work |
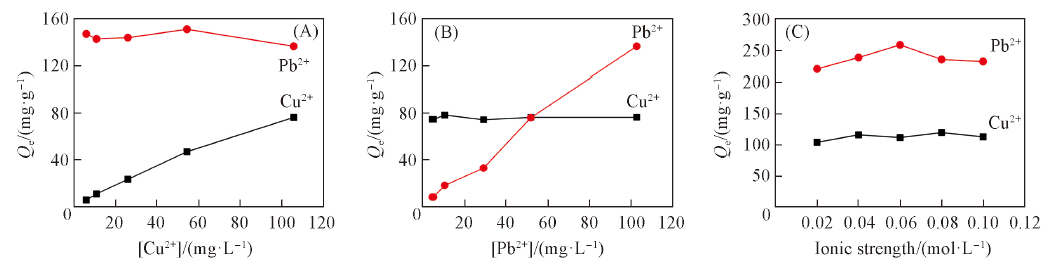
Fig.7 Variation of adsorption capacities of hydrophilic luffa sponge in Cu2+/Pb2+ mixture(20 oC, pH=5.00) with [Cu2+] concentration([Pb2+]=100 mg/L)(A), [Pb2+] concentration([Cu2+]=100 mg/L)(B) and ionic strength(c0=100 mg/L)(C)
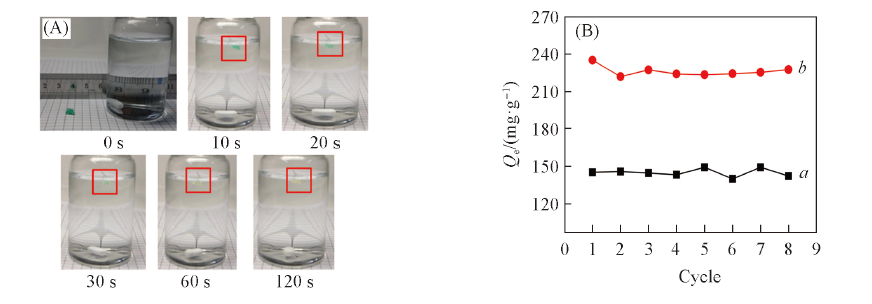
Fig.8 Desorption procedure of hydrophilic luffa sponge for Cu2+(20 oC, c0=100 mg/L, pH=1.00)(A) and adsorption capacities of hydrophilic luffa sponge for Cu2+(a) and Pb2+(b) at different regeneration cycles(20 oC, c0=100 mg/L, pH=5.00 for adsorption and pH=1.00 for desorption)(B)
| [1] | He J. S., Chen J. P., Bioresour Technol., 2014, 160, 67—78 |
| [2] | Mohanty K., Das D., Biswas M. N., Chem. Eng. J., 2005, 115, 121—131 |
| [3] | Veglio F., Beolchini F., Hydrometallurgy., 1997, 44, 301—316 |
| [4] | Kumar U., Bandyopadhyay M., Bioresour Technol., 2006, 97, 104—109 |
| [5] | Babel S., Kurniawan T. A., J. Hazard Mater., 2003, 97, 219—243 |
| [6] | Akhtar N, Iqbal J, Iqbal M., J. Hazard Mater., 2004, 108, 85—94 |
| [7] | Mallampati R., Li X. J., Adin A., Vaiyaveettil S., ACS Sustain Chem. Eng., 2015, 3, 1117—1124 |
| [8] | Li K., Wang Y. W., Huang M., Yan H., Yang H., Xiao S. J., Li A. M., J. Colloid Interface Sci., 2015, 455, 261—270 |
| [9] | Yan H., Yang L. Y., Yang Z., Yang H., Li A. M., Cheng R. S., J. Hazard Mater., 2012, 229, 371—380 |
| [10] | Cai T., Yang Z., Li H. J., Yang H., Li A. M., Cheng R. S., Cellulose,2013, 20, 2605—2614 |
| [11] | Zhang W. X., Yan H., Li H. J., Jiang Z. W., Dong L., Kan X. W., Yang H., Li A. M., Cheng R. S., Chem. Eng. J., 2011, 168, 1120—1127 |
| [12] | Yang R., Li H. J., Huang M., Yang H., Li A. M., Water Res., 2016, 95, 59—89 |
| [13] | Siqueira G., Bras J., Dufresne A., Bioresources,2010, 5, 727—740 |
| [14] | Akhtar N., Saeed A., Iqbal M., Bioresour. Technol., 2003, 88, 163—165 |
| [15] | Iqbal M., Edyvean R. G. J., Chemosphere,2005, 61, 510—518 |
| [16] | Akhtar N., Iqbal M., Zafar S. I., Iqbal J., J. Environ. Sci., 2008, 20, 231—239 |
| [17] | Laidani Y., Hanini S., Henini G., Impact of Integrated Clean Energy on the Future of the Mediterranean Environment,2011, 6, 381—388 |
| [18] | Henini G., Laidani Y., Souahi F., Hanini S., Energy Procedia, 2012, 18, 395—403 |
| [19] | Gupta V. K., Agarwal S., Singh P., Pathania D., Carbohydrate Polymers, 2013, 98, 1214—1221 |
| [20] | Liu Z., Pan Y., Shi K., Wang W., Peng C., Li W., Sha D., Wang Z., Ji X. Y., Carbohydrate Polymers, 2016, 147, 178—187 |
| [21] | Seki Y., Sever K., Erden S., Sarikanat M., Neser G., Ozes C., J. Appl. Polym. Sci., 2012, 123, 2330—2337 |
| [22] | Tserki V., Zafeiropoulos N. E., Simon F., Panayiotou C., Compos. Part A: Appl. Sci. Manuf., 2005, 36, 1110—1118 |
| [23] | Toth A., Faix O., Rachor G., Bertoti I., Szekely T., Appl. Surf. Sci., 1993, 72, 209—213 |
| [24] | Tanobe V. O. A., Sydenstricker T. H. D., Munaro M., Amico S. C., Polym. Test, 2005, 24, 474—482 |
| [25] | Xiong J. Q., Jiao C. L., Li C. M., Zhang D. S., Lin H., Chen Y. Y., Cellulose,2014, 21, 3073—3087 |
| [26] | Sun G., Shi W. X., Ind. Eng. Chem. Res., 1998, 37, 1324—1328 |
| [27] | Pan Y. X., Liu Z., Wang W. C., Peng C., Shi K., Ji X. L., J. Mater. Chem. A, 2016, 4, 2537—2549 |
| [28] | Zhao L. L., Liu H. R., Wang F. W., Zeng L., J. Mater. Chem. A, 2014, 2, 7065—7074 |
| [29] | Gupta H., Gogate PR., Ultrason Sonochem., 2016, 30, 113—122 |
| [30] | Chen Y., Q., Chen L. B., Bai H., Li L., J. Mater. Chem. A, 2013, 1,1992—2001 |
| [31] | Owamah H. I., J. Mater. Cycles Waste Manag., 2014, 16, 347—358 |
| [32] | Sheikhi A., Safari S., Yang H., van de Ven T. G. M.,ACS Appl. Mater. Interfaces, 2015, 7, 11301—11308 |
| [33] | Peng X. W., Zhong L. X., Ren J. L., Sun R. C., J. Agric. Food Chem., 2012, 60, 3909—3916 |
| [1] | LIU Yunhong, PENG Xinyan. Preparation and Property of A Novel Hemoperfusion Adsorbent For Protein-bound Uremic Toxins [J]. Chem. J. Chinese Universities, 2021, 42(6): 1952. |
| [2] | ZHAO Jie,SONG Qiang,GUO Xiao,WU Fei,TIAN Haochuan,LU Zhaowei. Synthesis of SnNb2O6 Nanoplates and Its Application for Danofloxacin Adsorption† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1121. |
| [3] | REN Jing, WANG Shugang, LI Yanchun, YANG Qingbiao, SONG Yan, LI Yaoxian. Preparation of AOPAN@PAN Coaxial Nanofiber Membrane and It’s Adsorption Property† [J]. Chem. J. Chinese Universities, 2018, 39(4): 825. |
| [4] | GONG Mei, ZHANG Li, HUANG Di, DU Jingjing, ZHOU Minbing, LI Yubao, XIE Huiqi. Grafting Modification and Thermosensitive Properties of Polyurethane Microspheres† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1843. |
| [5] | NING Yu, CAI Wen-Sheng, SHAO Xue-Guang. Simultaneous Determination of Mercury(Ⅱ) and Silver(Ⅰ) Ions in Water by Near-Infrared Spectroscopy with Preconcentration by Thiol-functionalized Polysilsesquioxane Microspheres [J]. Chem. J. Chinese Universities, 2012, 33(04): 673. |
| [6] | ZHANG Xia1*, ZHAO Yue1, ZHOU Chun-Bin2, SUN Ting1. Adsorption of Coordination Compound of Ag(I) on TiO2 Nanoparticles [J]. Chem. J. Chinese Universities, 2009, 30(1): 121. |
| [7] | LI Ji-Hong1,2, ZHANG Yuan-Wei1, YANG Mei1, ZHANG Jing1, MA Yi1, YUAN Zhi1*. Preparation of Adsorbents for Oligopeptide and Adsorption Mechanism [J]. Chem. J. Chinese Universities, 2008, 29(6): 1159. |
| [8] | WEI Zhong, HUANG Wei, LI Ji-Hong, YUAN Zhi*. Adsorbent Structure Design and Adsorption Mechanism by Computer Simulation [J]. Chem. J. Chinese Universities, 2007, 28(9): 1735. |
| [9] | GUO Xue-Jun, CHEN Fu-Hua . Elimination of As(Ⅲ) from Groundwater by Bead Cellulose Adsorbent Loaded with Fe(β-FeOOH) [J]. Chem. J. Chinese Universities, 2005, 26(7): 1258. |
| [10] | WANG Hui-Yan, HOU Guang-Hui, YU Mei, YUAN Zhi, LIU Bin, ZHAO Cheng-Mei. Adsorbents for Endotoxin Based on Chitosan Matrix [J]. Chem. J. Chinese Universities, 2005, 26(4): 680. |
| [11] | ZHANG Jin-Rong, CHEN Yan-Li, ZHANG Jing-Ze, GAO Ju, SHI Rong-Fu, YANG Yi-Zhong, SHI Zuo-Qing, WANG Chun-Hong. Preparation and Sieving Property of Adsorbing Resin with Homogeneous Pore Diameter and Controllable Large Pore [J]. Chem. J. Chinese Universities, 2005, 26(4): 765. |
| [12] | CHU Jia-Qiang, ZHANG Wei-Hua, YU Yao-Ting, ZHU Bo-Ru, CHEN Chang-Zhi . In Vitro Studies of the Immunoadsorbent for Removal of IgA in IgA-nephropathy (Ⅲ) [J]. Chem. J. Chinese Universities, 2004, 25(8): 1454. |
| [13] | WANG Hong, ZHANG Ming-Ming, YANG Mei, L� Jian, YUAN Zhi, HE Bing-Lin, LIU Bin, ZHAO Cheng-Mei, SHEN Bin . Investigations on the Adsorbents for Uremic Middle Molecular Toxins(Ⅲ)——Crosslinked Chitosan Bases Modified by Acidic Amino Acids [J]. Chem. J. Chinese Universities, 2004, 25(3): 474. |
| [14] | LIU Tao, HOU Guang-Hui, YU Mei, YUAN Zhi, LIU Bin, ZHAO Cheng-Mei, SHEN Bin, WANG Yan-Ming, WANG Shen-Qi . Hemperfusion Adsorbents With Lysine as Ligand for Removing of Endotoxin [J]. Chem. J. Chinese Universities, 2004, 25(12): 2284. |
| [15] | CHU Jia Qiang, YU Yao Ting, ZHANG Wei Hua, CHEN Jian Jun, ZHU Bo Ru, LIU Jing Duo . In vitro Studies on the Immunoadsorbent for Removal of IgA in IgA-Nephropathy (Ⅱ) [J]. Chem. J. Chinese Universities, 2003, 24(4): 636. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||