

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (2): 386.doi: 10.7503/cjcu20140908
• Polymer Chemistry • Previous Articles Next Articles
MA Ying, ZHANG Heng, YUAN Shiling*( )
)
Received:2014-10-11
Online:2015-02-10
Published:2015-01-06
Contact:
YUAN Shiling
E-mail:shilingyuan@sdu.edu.cn
Supported by:CLC Number:
TrendMD:
MA Ying, ZHANG Heng, YUAN Shiling. Hydration Structure of Partially Hydrolyzed Preformed Particle Gel†[J]. Chem. J. Chinese Universities, 2015, 36(2): 386.
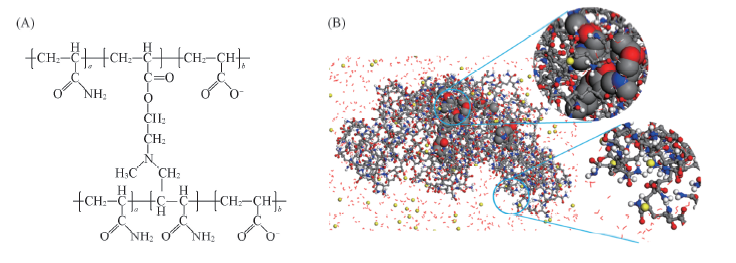
Fig.1 Scheme of cross-linked partially hydrolyzed polyacrylamide and conformations of simulation (A) Scheme of cross-linked partially hydrolyzed polyacrylamide; (B) initial conformation of mutually linked polymer network for molecular dynamics(MD) simulation with enlarged hydrophobic linker(in CPK model) and hydrophilic chains.
| Group | σ/nm | ε/(kJ·mol-1) | q/e |
|---|---|---|---|
| CH3 | 0.379 | 0.753 | 0 |
| CH2 | 0.395 | 0.586 | 0 |
| CH | 0.423 | 0.544 | 0 |
| C(CONH2) | 0.336 | 0.406 | 0.38 |
| O(CONH2) | 0.263 | 1.725 | -0.38 |
| N(CONH2) | 0.298 | 0.877 | -0.48 |
| H(CONH2) | 0 | 0 | 0.24 |
| C(COO-) | 0.336 | 0.406 | 0.27 |
| O(COO-) | 0.263 | 1.725 | -0.635 |
Table 1 Force field parameters for PPG used in this work*
| Group | σ/nm | ε/(kJ·mol-1) | q/e |
|---|---|---|---|
| CH3 | 0.379 | 0.753 | 0 |
| CH2 | 0.395 | 0.586 | 0 |
| CH | 0.423 | 0.544 | 0 |
| C(CONH2) | 0.336 | 0.406 | 0.38 |
| O(CONH2) | 0.263 | 1.725 | -0.38 |
| N(CONH2) | 0.298 | 0.877 | -0.48 |
| H(CONH2) | 0 | 0 | 0.24 |
| C(COO-) | 0.336 | 0.406 | 0.27 |
| O(COO-) | 0.263 | 1.725 | -0.635 |
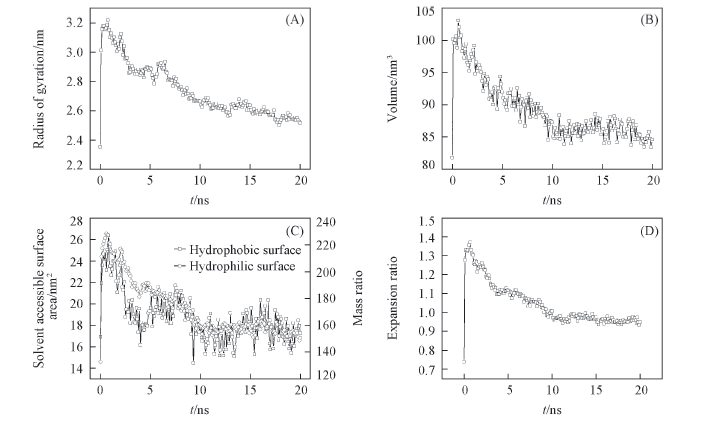
Fig.2 Conformation change of PPG during swelling (A) Radius of gyration; (B) volume; (C) hydrophilic and hydrophobic solvent access surface area; (D) expansion ratio.
| Certain atom of hydrophilic groups | 105 Diffusion coefficient, D/(cm2·s-1) | Residence time, τr/ps |
|---|---|---|
| O(COO-) | 2.95 | 13.78 |
| O(CONH2) | 3.25 | 6.58 |
| N(CONH2) | 3.50 | 10.71 |
| Bulk water[ | 3.58 | 4.40 |
Table 2 Dynamic properties of water around certain atoms of hydrophilic groups
| Certain atom of hydrophilic groups | 105 Diffusion coefficient, D/(cm2·s-1) | Residence time, τr/ps |
|---|---|---|
| O(COO-) | 2.95 | 13.78 |
| O(CONH2) | 3.25 | 6.58 |
| N(CONH2) | 3.50 | 10.71 |
| Bulk water[ | 3.58 | 4.40 |
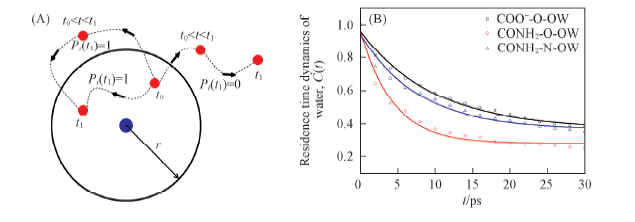
Fig.4 Residence time of water around certain atoms of hydrophilic groups (A) Illustration of definition of Pi, Pi(t1)=1 when water molecule in the first shell at t0 and t1. The location of water molecule during t0—t1 does not count; (B) time correlation functions of water around certain atoms of hydrophilic groups.
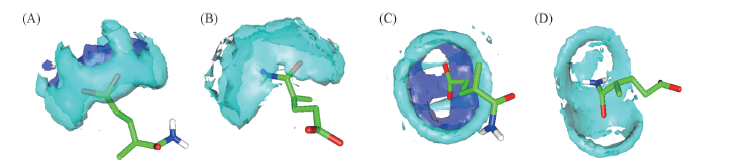
Fig.5 Spatial distributions of water and ions around certain atoms of hydrophilic groups(A, C) Spatial distribution of water and Na+ around —COO-; (B, D) spatial distribution of water around CONH2
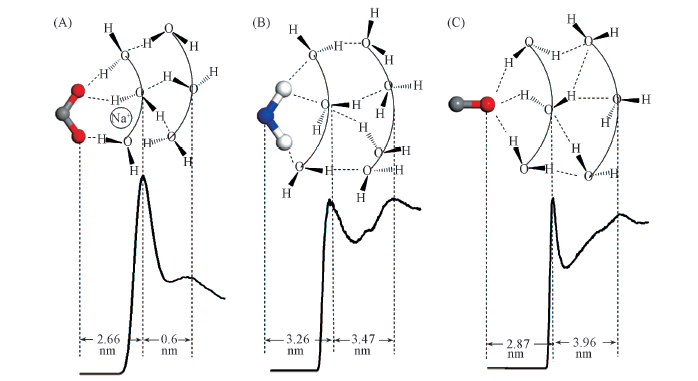
Fig.6 Radial distribution functions between certain atoms of hydrophilic groups and water molecules and scheme of hydrogen bond structure in hydration shells around them (A) gCOO-OW(r); (B) gN(CONH2)-OW(r); (C) gO(CONH2)-OW(r).
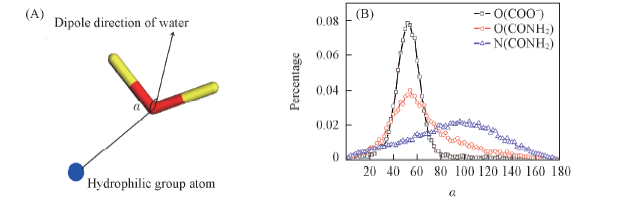
Fig.7 Definitions of α(A) and dipole orientation distribution of water molecules in the first hydration shell of certain atoms of hydrophilic groups(B)
| Certain atom of hydrophilic groups | Number of H bonds | Life time/ps | Dipole reorientation residence time, τμ/ps |
|---|---|---|---|
| O(COO-) | 1.64 | 10.20 | 6.17 |
| O(CONH2) | 1.33 | 1.75 | 4.68 |
| N(CONH2) | 1.14 | 1.11 | 4.28 |
Table 3 Hydrogen bond properties around certain atoms of hydrophilic groups
| Certain atom of hydrophilic groups | Number of H bonds | Life time/ps | Dipole reorientation residence time, τμ/ps |
|---|---|---|---|
| O(COO-) | 1.64 | 10.20 | 6.17 |
| O(CONH2) | 1.33 | 1.75 | 4.68 |
| N(CONH2) | 1.14 | 1.11 | 4.28 |
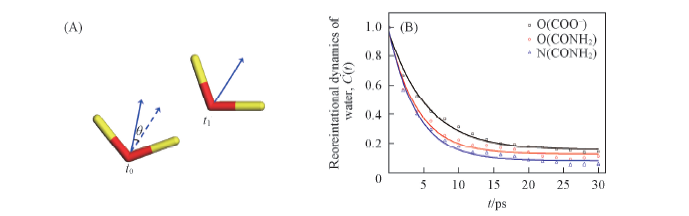
Fig.9 Illustration of angle between water dipole vectors(A) and reorientation autocorrelation functions of water around certain atoms of hydrophilic groups(B)
| [1] | Xiao L., Guo Y., Wang R., Lv S., Zhang Y. L., Luo Y. F., Petroleum Geology and Engineering, 2009, 23(6), 99—102 |
| 、(肖磊, 郭艳, 王锐, 吕帅, 张伊琳, 罗艳芳. 石油地质与工程, 2009, 23(6), 99—102) | |
| [2] | Deng S. F., Wei F. L., Wu M., Liang J., Advances in Fine Petrochemicals, 2011, 12(9), 17—20 |
| 、(邓生富, 魏发林, 吴蒙, 梁杰. 精细石油化工进展, 2011, 12(9), 17—20) | |
| [3] | Zhou Y. X., Hou T. J., Guo J. H., Sun J., Li X. D., Wang X., Zhang B., Advances in Fine Petrochemicals, 2005, 5(12), 22—2 |
| 、4(周亚贤, 侯天江, 郭建华, 孙举, 李旭东, 王旭, 张滨. 精细石油化工进展, 2005, 5(12), 22—24) | |
| [4] | Liu Y. Y., Chen P. K., Luo J. H., Zhou G., Jiang B., Acta Phys. Chim. Sin., 2010, 26(11), 2907—2914 |
| 、(刘艳艳, 陈攀科, 罗健辉, 周歌, 江波. 物理化学学报, 2010, 26(11), 2907—2914) | |
| [5] | Jia H., Pu W. F., Zhao J. Z., Liao R., Energ. Fuel., 2011, 25(2), 727—736 |
| [6] | Jia H., Ren Q., Zhao J., Energ. Fuel., 2014, 28(11), 6735—6744 |
| [7] | Jia H., Zhao J. Z., Jin F. Y., Pu W. F., Li Y. M., Li K. X., Li J. M., Ind. Eng. Chem. Res., 2012, 51(38), 12155—12166 |
| [8] | Bai B. J., Li L. X., Liu Y. Z., Liu H., Wang Z. G., You C. M., SPE Reserv. Eval. Eng., 2007, 10(4), 415—422 |
| [9] | Bai B. J., Zhang H., SPE J., 2011, 16(2), 388—400 |
| [10] | Wang J., Liu H. Q., Wang Z. L., Xu J., Yuan D. Y., J. Petrol. Sci. Eng., 2013, 112, 248—257 |
| [11] | Ma J., Liang B., Cui P., Dai H., Huang R., Polymer, 2003, 44(4), 1281—1286 |
| [12] | Feng Y., Grassl B., Billon L., Khoukh A., François J., Polym. Int., 2002, 51(10), 939—947 |
| [13] | Wang Y. Y., Dai Y. H., Zhang L., Luo L., Chu Y. P., Zhao S., Li M. Z., Wang E. J., Yu J. Y., Macromolecules, 2004, 37(8), 2930—2937 |
| [14] | Feng Y., Billon L., Grassl B., Bastiat G., Borisov O., François J., Polymer, 2005, 46(22), 9283—9295 |
| [15] | Feng Y., Billon L., Grassl B., Khoukh A., François J., Polymer, 2002, 43(7), 2055—2064 |
| [16] | Xue W., Hamley I. W., Castelletto V., Olmsted P. D., Eur. Polym. J., 2004, 40(1), 47—56 |
| [17] | Shashkina Y. A., Zaroslov Y. D., Smirnov V., Philippova O., Khokhlov A., Pryakhina T., Churochkina N., Polymer, 2003, 44(8), 2289—2293 |
| [18] | Sheng Y. Z., Yang H., Li J. Y., Sun M., Chem. Res. Chinese Universities, 2013, 29(4), 788—792 |
| [19] | Kong C. P., Zhang H. X., Zhao Z. X., Zhong Q. C., Chem. Res. Chinese Universities, 2013, 29(3), 545—550 |
| [20] | Netz P. A., Dorfmüller T., J. Phys. Chem. B, 1998, 102(25), 4875—4886 |
| [21] | Chen P., Yao L., Liu Y., Luo J., Zhou G., Jiang B., J. Mol. Model., 2012, 18(7), 3153—3160 |
| [22] | Yuan R., Li Y., Li C., Fang H., Wang W., Colloid. Surface. A, 2013, 434, 16—24 |
| [23] | Wang H. X., Yao L., Ding B., Luo J. H., Zhou G., Jiang B., Chem. J. Chinese Universities, 2013, 34(5), 1295—1302 |
| (王惠厦, 姚林, 丁彬, 罗健辉, 周歌, 江波.高等学校化学学报, 2013, 34(5), 1295—1302) | |
| [24] | Schuler L. D., Daura X., van Gunsteren W. F., J. Comput. Chem., 2001, 22(11), 1205—1218 |
| [25] | Hess B., Kutzner C., van der Spoel D., Lindahl E., J. Chem. Theory Comp., 2008, 4(3), 435—477 |
| [26] | Oldiges C., Tönsing T., Phys. Chem. Chem. Phys., 2002, 4(9), 1628—1636 |
| [27] | Sulatha M. S., Natarajan U., Ind. Eng. Chem. Res., 2011, 50(21), 11785—11796 |
| [28] | Brandsen H., Postma J., van Gunstern E., Hermans J., Pullman B., Intermolecular Force, Reidel, Dordrecht, 1981 |
| [29] | Essmann U., Perera L., Berkowitz M. L., Darden T., Lee H., Pedersen L. G., J. Chem. Phys., 1995, 103(19), 8577—8593 |
| [30] | Berendsen H. J., Postma J. P. M., van Gunsteren W. F., DiNola A., Haak J., J. Chem. Phys., 1984, 81(8), 3684—3690 |
| [31] | Hess B., Bekker H., Berendsen H. J., Fraaije J. G., J. Comput. Chem., 1997, 18(12), 1463—1472 |
| [32] | Liu S. R., Kang W. L., Bai B. J., Zhao H. Y., Meng L. W., Journal of China University of Petroleum(Natural Science), 2013, 37(2), 153—157(刘述忍, 康万利, 白宝君, 赵昊阳, 孟令伟. 中国石油大学学报: 自然科学版, 2013, 37(2), 153—157) |
| [33] | Zhang H., Wang H., Lin C.G., Wang L., Yuan S. L.,Acta Chim. Sin., 2013, (4), 649—656 |
| (张恒, 王华, 蔺存国, 王利, 苑世领. 化学学报, 2013, (4), 649—656) | |
| [34] | Shao Q., He Y., White A. D., Jiang S., J. Phys. Chem. B, 2010, 114(49), 16625—16631 |
| [35] | He Y., Hower J., Chen S., Bernards M. T., Chang Y., Jiang S., Langmuir, 2008, 24(18), 10358—10364 |
| [36] | He Y., Chang Y., Hower J. C., Zheng J., Chen S., Jiang S., Phys. Chem. Chem.Phys., 2008, 10(36), 5539—5544 |
| [37] | Hower J. C., He Y., Bernards M. T., Jiang S., J. Chem. Phys., 2006, 125(21), 214704 |
| [38] | Rasaiah J. C., Noworyta J. P., Koneshan S., J. Am. Chem. Soc., 2000, 122(45), 11182—11193 |
| [39] | Shao Q., Zhou J., Lu L., Lu X., Zhu Y., Jiang S., Nano Lett., 2009, 9(3), 989—994 |
| [40] | Zhou J., Lu X., Wang Y., Shi J., Fluid Phase Equilibr., 2002, 194, 257—270 |
| [41] | Shao Q., Huang L., Zhou J., Lu L., Zhang L., Lu X., Jiang S., Gubbins K. E., Shen W., Phys. Chem. Chem. Phys., 2008, 10(14), 1896—1906 |
| [42] | Bandyopadhyay S., Chakraborty S., Bagchi B., J. Am. Chem. Soc., 2005, 127(47), 16660—16667 |
| [43] | Guardia E., Martí J., García-Tarrés L., Laria D., J. Mol. Liq., 2005, 117(1), 63—67 |
| (Ed.: D, Z) |
| [1] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [2] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [3] | TIAN Yao,ZHANG Chunquan,WANG Wenzhe,ZHOU Yingfang,LU Yitong,ZHANG Peng,JIA Zhenfu,ZHOU Chengyu,CHEN Shilan. Preparation of Polyrotaxane Cross-linking Agent with “Pulley” Effect and Its Potential Application in Swelling Grain Used as Profile Control and Water Plugging Agent† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2098. |
| [4] | YE Hui, LIU Yabo, JIA Yuxi. Numerical Simulation of Swelling and Drug Release Processes for Weak Polyelectrolyte Hydrogels† [J]. Chem. J. Chinese Universities, 2018, 39(4): 817. |
| [5] | GAO Tingting, LI Zhiying, GAO Ge, LIU Fengqi. Mechanical Strength and Swelling Behavior of Fatty Alcohol Polyoxyethylene Acrylate Hydrophobic Associated Hydrogels† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1744. |
| [6] | WU Lixiang, CAI Zhibin, CHEN Xiaolin, LIU Lifen, ZHU Lifang, GAO Congjie. Stability of a Novel Poly(amide-urea-imide) Composite Reverse Osmosis Membrane† [J]. Chem. J. Chinese Universities, 2015, 36(4): 765. |
| [7] | TANG Li, LUO Lan, FANG Hong-Bo, ZONG Hua, ZHANG Lei, ZHANG Lu, ZHAO Sui. Dilational Rheology Properties of Branch-performed Particle Gel by Relaxation Measurements [J]. Chem. J. Chinese Universities, 2013, 34(6): 1434. |
| [8] | WANG Dan, BAI Yin-Juan, HE Gui-Qiang, ZHANG Yu-Cheng, GONG Yong-Kuan, ZHANG Shi-Ping. Synthesis, Characterization and Properties of a Novel Unsaturated Polyphosphoester [J]. Chem. J. Chinese Universities, 2013, 34(6): 1555. |
| [9] | XU Yu, CUI Ying-Lu, ZHENG Qing-Chuan, ZHANG Hong-Xing, SUN Chia-Chung. Theoretical Studies on Interaction Modes Between Human GSTP1*B and Inhibitors [J]. Chem. J. Chinese Universities, 2013, 34(5): 1226. |
| [10] | SHAN Ning, LIAN Wen-Hui, WANG Bin-Bin, SUN Yuan-Yuan, ZHENG Wen-Qi, YU Miao, SHI Tong-Shun*. Synthesis and Characterization of a New Tailed Histidine-Linked Porphyrin [J]. Chem. J. Chinese Universities, 2011, 32(12): 2733. |
| [11] | LI Jian-Jun, ZHANG Cheng-Liang, DING Shu-Jiang, QU Xiao-Zhong, YANG Zhen-Zhong*. Template Synthesis of Composite Hollow Spheres [J]. Chem. J. Chinese Universities, 2009, 30(9): 1904. |
| [12] | WU Wen, WANG Dong-Sheng, WANG Li-Qun*. Synthesis and Characterization of Fast pH-Responsive Silk Sericin/Poly(methacrylic acid) Interpenetrating Polymer Network Hydrogel [J]. Chem. J. Chinese Universities, 2009, 30(4): 830. |
| [13] | QI Yan-Feng1, GAO Xue-Feng1*, HUANG Xu-Ri2. Theoretical Mutation Design of Active Agent of Eryhropoietin and Its Receptor [J]. Chem. J. Chinese Universities, 2008, 29(3): 615. |
| [14] | LI Qian, CHEN Xin-Fu, ZHANG Zheng-Pu*. Preparation of Carboxyl Monodispersed Colour Microspheres [J]. Chem. J. Chinese Universities, 2008, 29(2): 399. |
| [15] | WANG Fei-Jun1, SHAO Zi-Qiang1*, WANG Wen-Jun1, LÜ Shao-Yi1, FENG Zeng-Guo1, LIAO Bing2. Effect of Alkali Agents on Molecular Structure of PAC and Drilling-mud Fluid Loss [J]. Chem. J. Chinese Universities, 2008, 29(11): 2326. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||