

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (9): 1857.doi: 10.7503/cjcu20190158
• Analytical Chemistry • Previous Articles Next Articles
YU Qiongwei,ZHENG Feng,FANG Kaimin,FENG Yuqi( )
)
Received:2019-03-15
Online:2019-09-10
Published:2019-07-12
Contact:
FENG Yuqi
E-mail:yqfeng@whu.edu.cn
Supported by:CLC Number:
TrendMD:
YU Qiongwei, ZHENG Feng, FANG Kaimin, FENG Yuqi. Preparation of Zirconia Deposited Silica Stationary Phase and Its Application to Hydrophilic-interaction Liquid Chromatography†[J]. Chem. J. Chinese Universities, 2019, 40(9): 1857.
| Sample | Surface area/<break/>(m2·g-1) | Pore volume/<break/>(cm3·g-1) | Pore diameter/<break/>nm |
|---|---|---|---|
| SiO2 | 287 | 1.1 | 11 |
| ZrO2/SiO2 | 260 | 1.0 | 11 |
| ZrO2 | 14 | 0.07 | 18 |
| Sample | Surface area/<break/>(m2·g-1) | Pore volume/<break/>(cm3·g-1) | Pore diameter/<break/>nm |
|---|---|---|---|
| SiO2 | 287 | 1.1 | 11 |
| ZrO2/SiO2 | 260 | 1.0 | 11 |
| ZrO2 | 14 | 0.07 | 18 |
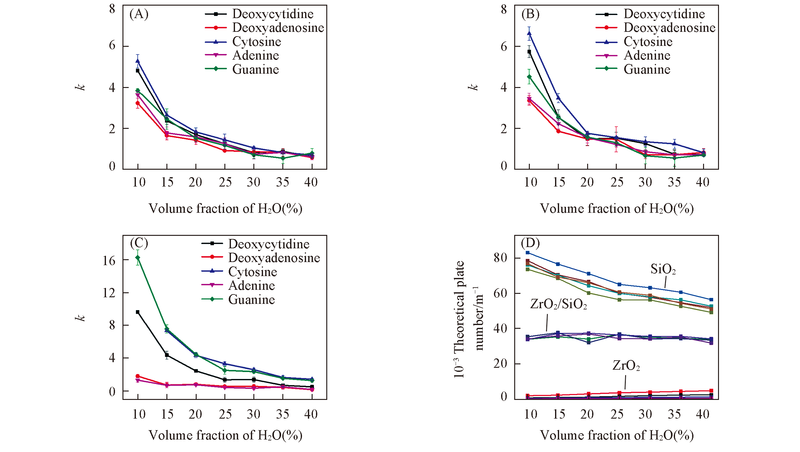
Fig.4 Plots of k and theoretical plate numbers vs. H2O volume fraction in mobile phase(A) SiO2; (B) ZrO2/SiO2; (C) ZrO2; (D) theoretical plate numbers of tested analytes on different columns.Mobile phase: ACN-20 mmol/L NH4Ac(pH=6.8); flow rate: 1.0 mL/min; UV detection wavelength: 254 nm.
| Tested analyte | Correlation coefficient on SiO2 | Correlation coefficient on ZrO2/SiO2 | Correlation coefficient on ZrO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eq.(1) | Eq.(2) | Eq.(3) | Eq.(1) | Eq.(2) | Eq.(3) | Eq.(1) | Eq.(2) | Eq.(3) | |
| Deoxycytidine | 0.9179 | 0.9537 | 0.9999 | 0.8947 | 0.9866 | 0.9987 | 0.9397 | 0.9980 | 0.9984 |
| Deoxyadenosine | 0.9147 | 0.9179 | 0.9998 | 0.8883 | 0.9839 | 0.9981 | 0.9771 | 0.98840 | 0.9934 |
| Cytosine | 0.9015 | 0.9147 | 0.9998 | 0.8546 | 0.9670 | 0.9926 | 0.9693 | 0.9988 | 0.9987 |
| Adenine | 0.8936 | 0.9015 | 0.9999 | 0.8662 | 0.9751 | 0.9987 | 0.9670 | 0.9919 | 0.9930 |
| Guanine | 0.9090 | 0.8936 | 0.9998 | 0.8781 | 0.9801 | 0.9985 | 0.9207 | 0.9948 | 0.9991 |
| Tested analyte | Correlation coefficient on SiO2 | Correlation coefficient on ZrO2/SiO2 | Correlation coefficient on ZrO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eq.(1) | Eq.(2) | Eq.(3) | Eq.(1) | Eq.(2) | Eq.(3) | Eq.(1) | Eq.(2) | Eq.(3) | |
| Deoxycytidine | 0.9179 | 0.9537 | 0.9999 | 0.8947 | 0.9866 | 0.9987 | 0.9397 | 0.9980 | 0.9984 |
| Deoxyadenosine | 0.9147 | 0.9179 | 0.9998 | 0.8883 | 0.9839 | 0.9981 | 0.9771 | 0.98840 | 0.9934 |
| Cytosine | 0.9015 | 0.9147 | 0.9998 | 0.8546 | 0.9670 | 0.9926 | 0.9693 | 0.9988 | 0.9987 |
| Adenine | 0.8936 | 0.9015 | 0.9999 | 0.8662 | 0.9751 | 0.9987 | 0.9670 | 0.9919 | 0.9930 |
| Guanine | 0.9090 | 0.8936 | 0.9998 | 0.8781 | 0.9801 | 0.9985 | 0.9207 | 0.9948 | 0.9991 |
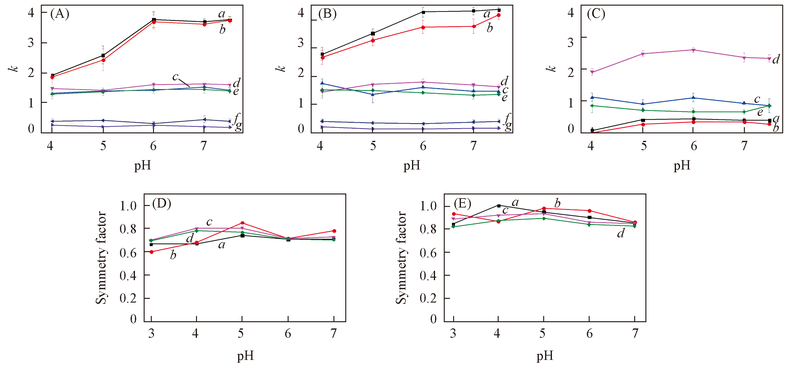
Fig.5 Effects of mobile phase pH values on retention factors(k) and symmetry factors of tested analytes(A) SiO2; (B) ZrO2/SiO2; (C) ZrO2; (D) effect of pH on the symmetry factor of tested analytes on SiO2 column; (E) effect of pH on the symmetry factor of tested analytes on ZrO2/SiO2 column. Mobile phase: ACN-20 mmol/L NH4Ac(80∶20, volume ratio). Flow rate: 1.0 mL/min; UV detection wavelength: 254 nm. (A)—(C) a. Proparanolol; b. berberine; c. melamine; d. deoxycytidine; e. adenine; f. benzoic acid; g. p-nitrobenzoic acid. (D) and (E) a. Propranolo; b. berberine; c. deoxycytidine; d. adenine.
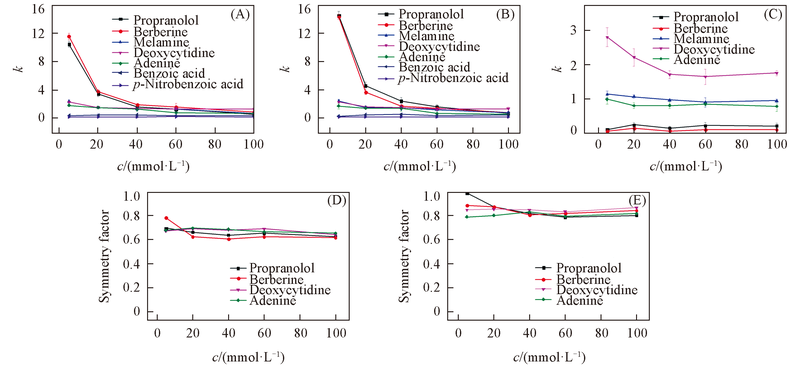
Fig.6 Influence of NH4Ac concentration on the retention factor(k) and symmetry factors of tested analytes(A) SiO2; (B) ZrO2/SiO2; (C) ZrO2; (D) effect of NH4Ac concentration on the symmetry factor of tested analytes on SiO2column; (E) effect of NH4Ac concentration on the symmetry factor of tested analytes on ZrO2/SiO2 column. Mobile phase: ACN-NH4Ac(pH=6.8)(80∶20, volume ratio). Flow rate: 1.0 mL/min; UV detection wavelength: 254 nm.
| ZrO2 related stationary phase | Interaction mechanism | Application | Ref. |
|---|---|---|---|
| Zirconia-based stationary phase cellulose | Ion-exchange interaction | Drug control | [45] |
| Tris(3,5-dimethylphenylcarbamate)-coatedzirconia | Chiral compounds | [46] | |
| Octadecyl coated zirconia stationary phase | Ion-exchange interaction | Parabens | [47] |
| N-Methylimidazolium functionalized ZrO2/SiO2-4 | Ion-exchange interaction | Inorganic and organic anions | [48] |
| Adenosine 5'-monophosphate modified ZrO2/SiO2 | Hydrogen-bonding, electrostatic and <br/>ion-exchange interaction | Acidic compounds | [49] |
| ZrO2/SiO2 | Adsorption and partition | Polar compounds | This work |
| ZrO2 related stationary phase | Interaction mechanism | Application | Ref. |
|---|---|---|---|
| Zirconia-based stationary phase cellulose | Ion-exchange interaction | Drug control | [45] |
| Tris(3,5-dimethylphenylcarbamate)-coatedzirconia | Chiral compounds | [46] | |
| Octadecyl coated zirconia stationary phase | Ion-exchange interaction | Parabens | [47] |
| N-Methylimidazolium functionalized ZrO2/SiO2-4 | Ion-exchange interaction | Inorganic and organic anions | [48] |
| Adenosine 5'-monophosphate modified ZrO2/SiO2 | Hydrogen-bonding, electrostatic and <br/>ion-exchange interaction | Acidic compounds | [49] |
| ZrO2/SiO2 | Adsorption and partition | Polar compounds | This work |
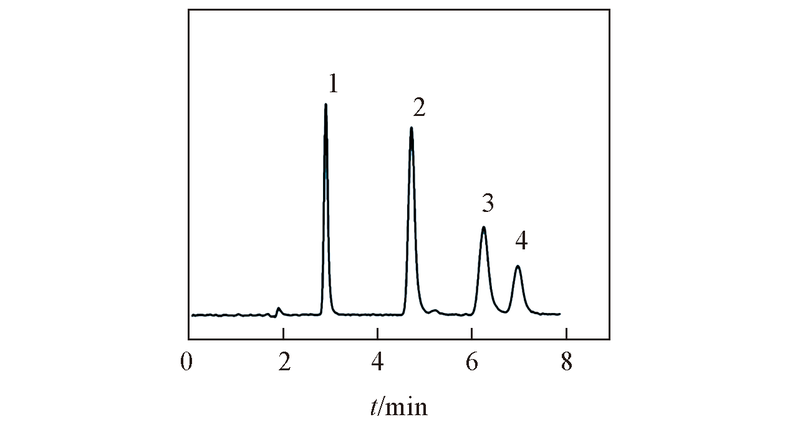
Fig.7 Separation of four deoxynucleosides on ZrO2/SiO2 columnMobile phase: ACN-20 mmol/L NH4Ac(pH=6.8)(80∶20, volume ratio). Flow rate: 1.0 mL/min; UV detection wavelength: 254 nm. Peak 1: thymidine; peak 2: 2'-deoxyadenosine; peak 3: 2'-deoxyguanosine; peak 4: 2'-deoxycytidine.
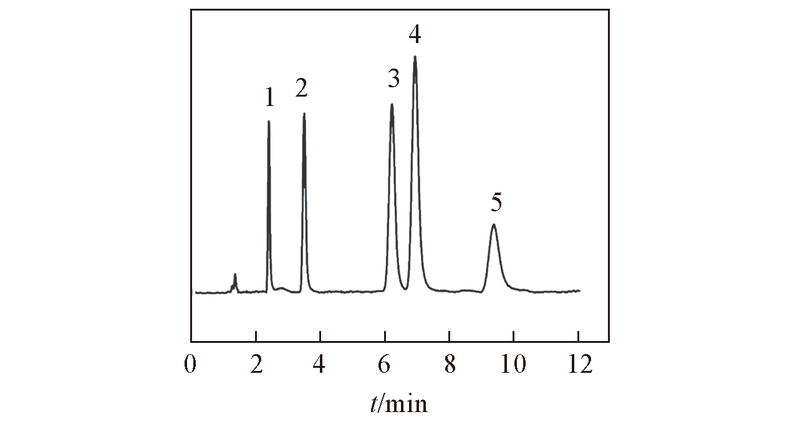
Fig.8 Separation of several basic analytes on ZrO2/SiO2 columnMobile phase: ACN-20 mmol/L NH4Ac(pH=6.8)(80∶20, volume ratio). Flow rate: 1.0 mL/min; UV detection wavelength: 254 nm. Peak 1: theophylline; peak 2: melamine; peak 3: ractopamine; peak 4 clenbuterol; peak 5: terbutaline.
| [1] | Peng X. T., Yuan B. F., Feng Y. Q., J. Sep. Sci.,2011, 34, 3123—3130 |
| [2] | Dejaegher B., Mangelings D., Heyden Y. V., J. Sep. Sci., 2008, 31(9), 1438—1448 |
| [3] | Alpert A. J., J. Chromatogr.A, 1990, 499, 177—196 |
| [4] | Zhu B. Y., Mant C. T., Hodges R. S., J. Chromatogr. A,1991, 548(1/2), 13—24 |
| [5] | Strege M. A., Anal. Chem., 1998, 70(13), 2439—2445 |
| [6] | Olsen B. A., J. Chromatogr. A, 2001, 913(1/2), 113—122 |
| [7] | Tolstikov V. V., Fiehn O., Anal. Biochem.,2002, 301(2), 298—307 |
| [8] | Karlsson G., Winge S., Sandberg H., J. Chromatogr. A,2005, 1092(2), 246—249 |
| [9] | Gianotti V., Chiurminatto U., Mazzucco E., J. Chromatogr. A,2008, 1185(2), 296—300 |
| [10] | Al-Rimawi F., Talanta, 2009, 79(5), 1368—1371 |
| [11] | Kawano S., Rapid Communications in Mass Spectrometry, 2009, 23(6), 907—914 |
| [12] | Dejaegher B., Heyden Y. V., J. Sep. Sci., 2010, 33(6/7), 698—715 |
| [13] | Jian W. Y., Edom R. W., Xu Y. D., J. Sep. Sci., 2010, 33(6/7), 681—697 |
| [14] | Fontanals N., Marce R. M., Borrull F., J. Chromatogr. A,2011, 1218(35), 5975—5980 |
| [15] | Churms S. C., J. Chromatogr. A, 1996, 720(1/2), 75—91 |
| [16] | Yoshida T., Anal. Chem., 1997, 69(15), 3038—3043 |
| [17] | Yoshida T., J. Biochem. Biophys. Methods, 2004, 60(3), 265—280 |
| [18] | Yang Y. Z., Boysen R. I., Hearn M. T. W., J. Chromatogr. A,2009, 1216(29), 5518—5524 |
| [19] | McNulty D. E., Annan R. S., Molecular & Cellular Proteomics,2008, 7(5), 971—980 |
| [20] | Bicker W., Wu J. Y., Yeman H., J. Chromatogr. A,2011, 1218(7), 882—895 |
| [21] | Kotoni D., D'Acquarica I., Ciogli A., J. Chromatogr. A,2012, 1232, 196—211 |
| [22] | Alpert A. J., Anal. Chem., 2008, 80(1), 62—76 |
| [23] | Mant C. T., Hodges R. S., J. Sep. Sci., 2008, 31(15), 2754—2773 |
| [24] | Lindner H., Sarg B., Helliger W., J. Chromatogr. A,1997, 782(1), 55—62 |
| [25] | Persson J., Hemstrom P., Irgum K., J. Sep. Sci., 2008, 31(9), 1504—1510 |
| [26] | Que A. H., Konse T., Baker A. G., Anal. Chem.,2000, 72(13), 2703—2710 |
| [27] | Que A. H., Novotny M. V., Anal. Chem.,2002, 74(20), 5184—5191 |
| [28] | Hosoya K., Hira N., Yamamoto K., Anal. Chem.,2006, 78(16), 5729—5735 |
| [29] | Jiang Z. J., Smith N. W., Ferguson P. D., J. Sep. Sci., 2009, 32(15/16), 2544—2555 |
| [30] | Jiang Z. J., Reilly J., Everatt B., J. Chromatogr. A,2009, 1216(12), 2439—2448 |
| [31] | Tijunelyte I., Babinot J., Guerrouache M., Polymer,2012, 53(1), 29—36 |
| [32] | Nawrocki J., Dunlap C., Li J., Zhao J., McNeff C. V., McCormick A., Carr P. W., J. Chromatogr. A,2004, 1028, 31—62 |
| [33] | Kucera R., Kovaǐíková P., Klivický M., Klimeš J., J. Chromatogr. A,2011, 1218(39), 6981—6986 |
| [34] | Kalafut P., Kuc era R., Klimes J., J. Chromatogr. A,2012, 1232, 242—247 |
| [35] | Song Z. H., Duan C. F., Shi M., Guan Y. F., J. Chromatogr. A,2017, 1522, 30—37 |
| [36] | Wang Q., Li J., Yang X., Talanta,2014, 129, 438—447 |
| [37] | Yu Q. W., Fang K. M., He X. M., Zheng J., Feng Y. Q., Chinese J. Chromatogr., 2018, 36(3), 237—244 |
| (余琼卫, 方凯敏, 何小梅, 郑杰, 冯钰锜.色谱,2018, 36(3), 237—244) | |
| [38] | Yu Q. W., Feng Y. Q., Progr.Chem., 2011, 23(6), 1211—1223 |
| (余琼卫, 冯钰锜.化学进展, 2011, 23(6), 1211—1223) | |
| [39] | He H. B., Yu Q. W., Feng Y. Q., Da S. L., J. Liq. Chromatogr. Related. Tech., 2009, 32, 468—482 |
| [40] | Alpert A. J., J. Chromatogr. A, 1990, 499, 177—196 |
| [41] | Scott R. P., Kucera P., J. Chromatogr. A,1978, 149, 93—110 |
| [42] | Jandera P., Anal. Chim. Acta, 2011, 692(1), 1—25 |
| [43] | Karatapanis A. E., Fiamegos Y. C., Stalikas C. D., J. Chromatogr. A,2011, 1218(20), 2871—2879 |
| [44] | Nawrocki J., Rigney M., McCormick A., J. Chromatogr. A,1993, 657(2), 229—282 |
| [45] | Radim K., Jaroslav S., J. Sep. Sci., 2005, 28, 1307—1314 |
| [46] | Cecilia B., Castells Peter W. C., Anal. Chem., 1999, 71, 3013—3021 |
| [47] | Paola D., Katia B., Maria Lucia C., Francesco C., Giovanni D., Luigi M., J. Sep. Sci., 2007, 30, 1125—1130 |
| [48] | Liang X. J., Chen Q. S., Liu X., Jiang S. X., J. Chromatogr. A,2008, 1182, 197—204 |
| [49] | Wang Q., Luo Z. Y., Ye M., Xu L., Shi Z. G., Xu L. Y., J. Chromatogr. A,2015, 1383, 58—69 |
| [1] | QIAO Junqin,LIANG Chao,CAO Zhaoming,LIAN Hongzhen. Retention Behavior of Oligonucleotides under System Containing Mixed Ion-pair Reagents by IP-RPLC† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1893. |
| [2] | YUAN Jian, ZHANG Bo, TANG Ming-Hui, LU Han-Feng, CHEN Yin-Fei. Comparison of ZrO2/MCM-41 and ZrO2/AC Catalysts in Hydrogen Transfer Reaction of Acetophenone [J]. Chem. J. Chinese Universities, 2012, 33(06): 1326. |
| [3] | YANG Jun-Jiao*. Preparation of Novel Mixed Packing Materils and Its Evaluation [J]. Chem. J. Chinese Universities, 2009, 30(5): 896. |
| [4] | CHU Bin, WANG Run-Wei, SHEN Qi-Hui, CHEN Lu, WAN Li-Feng, ZHU Guang-Shan, QIU Shi-Lun*. Preparation and Structure Characterization of Ordered Cubic Zr-doped Mesoporous Silica by EISA Method [J]. Chem. J. Chinese Universities, 2007, 28(8): 1428. |
| [5] | ZHAO Yi-Yang, WANG Hai-Ying, LI Xiang, YANG Yang, YANG Min, WANG Ce. Preparation of Sulfated Zirconia/Silica Complex Nanofibers by Electrospinning Method [J]. Chem. J. Chinese Universities, 2007, 28(2): 382. |
| [6] | ZHANG Yu-Juan, WANG Guo-Zhi, ZHANG Lei, DAI Hong-Xing*, HE Hong, ZI Xue-Hong. Synthesis, Characterization and Catalytic Properties of Three-dimensional Wormhole-like Mesoporous Ag2O/Ce0.6Zr0.35Y0.05O2 Nanoparticles in Methane Oxidation [J]. Chem. J. Chinese Universities, 2007, 28(10): 1929. |
| [7] | LI Yan, WANG Yu-He, XU Bo-Qing. Nano-structural Characters of Highly Active and Stable Ni/ZrO2 Catalyst for CO2 Reforming of Methane [J]. Chem. J. Chinese Universities, 2005, 26(7): 1325. |
| [8] | YU Qiong-Wei, SHI Zhi-Guo, DA Shi-Lu, FENG Yu-Qi. Preparation of Pyrenebutyric Acid-modified Zirconia-Magnesia Stationary Phase for High Performance Liquid Chromatography and Its Application to the Separation of Fullerenes [J]. Chem. J. Chinese Universities, 2005, 26(7): 1237. |
| [9] | MA Zhong-Yi, YANG Cheng, DONG Qing-Nian, WEI Wei, CHEN Jian-Gang, LI Wen-Huai, SUN Yu-Han . Adsorption and Reaction Behavior over Co Catalysts Supported by Different Zirconia Polymorphs [J]. Chem. J. Chinese Universities, 2005, 26(5): 902. |
| [10] | YAO Li-Feng, FENG Yu-Qi, DA Shi-Lu . Adsorption Mode of Di-dentate Ligand Modified Zirconia Stationary Phase Surface [J]. Chem. J. Chinese Universities, 2005, 26(2): 244. |
| [11] | LIU Ming-Fei, JIANG Yin-Zhu, GAO Jian-Feng, WANG Yan-Yan, MENG Guang-Yao. Synthesis of YSZ Film by Solid Single Source PE-MOCVD Method and Characterization [J]. Chem. J. Chinese Universities, 2005, 26(11): 1981. |
| [12] | DUN Hui-Juan, WEI Yu, SONG Xiu-Qin, CHEN Li-Ren. Surface Properties of Zirconia-based Supports for HPLC [J]. Chem. J. Chinese Universities, 2005, 26(11): 2040. |
| [13] | ZHANG Xin, XU Bo-Qing . Size Effect of Zirconia Nanoparticles in Au/ZrO2 Catalysts for 1,3-Butadiene Hydrogenation [J]. Chem. J. Chinese Universities, 2005, 26(1): 106. |
| [14] | YANG Jun-Jiao, ZUO Yu-Min . Preparation and Evaluation of Polymer-encapsulated Zirconia-based Packing Stationary Phase for HPLC [J]. Chem. J. Chinese Universities, 2005, 26(1): 35. |
| [15] | DUN Hui-Juan, LI Yong-Min, LIU Chun-Hui, WEI Yong-Ju, CHEN Li-Ren . Chiral Resolution of Naproxen and Its Derivatives on Chiral Stationary Phase of Cellulose Tris(3,5-dimethylphenylcarbamate)-Coated Zirconia [J]. Chem. J. Chinese Universities, 2004, 25(3): 451. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||