

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (7): 1390.doi: 10.7503/cjcu20180812
• Analytical Chemistry • Previous Articles Next Articles
HAN Mingxin, LI Fangtong, ZHANG Yan, DAI Yulin, ZHENG Fei*( ), YUE Hao*(
), YUE Hao*( )
)
Received:2018-12-03
Online:2019-07-10
Published:2019-07-12
Contact:
ZHENG Fei,YUE Hao
E-mail:zhengfei@ccucm.edu.cn;yuehao@sohu.com
Supported by:CLC Number:
TrendMD:
HAN Mingxin, LI Fangtong, ZHANG Yan, DAI Yulin, ZHENG Fei, YUE Hao. Biotransformation of Rare Protopanaxadiol Saponin by Human Intestinal Microflora†[J]. Chem. J. Chinese Universities, 2019, 40(7): 1390.
| Sample | Compd. | tR/min | Mw | MS, m/z | MS/MS fragment ion, m/z | Relative error/ (ng·mL-1) | Product |
|---|---|---|---|---|---|---|---|
| M1 | Rd | 26.187 | 991.5478 | 991.5443[M+HOOCH-H]- | 783.4929[M-H-Glc]-, | 3.53 | F2, Rg3, CK, |
| 621.4354[M-H-2Glc]-, | Rh2, PPD | ||||||
| 459.3819[M-H-3Glc]- | |||||||
| M2 | F2 | 31.421 | 829.4949 | 829.4947[M+HOOCH-H]- | 621.4308[M-H-Glc]-, | 0.24 | CK, PPD |
| 459.3821[M-H-2Glc]- | |||||||
| M3 | Rg3 | 32.693 | 829.4949 | 829.4884[M+HOOCH-H]- | 621.4311[M-H-Glc]-, | 7.84 | Rh2, PPD |
| 459.3824[M-H-2Glc]- | |||||||
| M4 | CK | 35.411 | 667.4421 | 621.4369[M-H]-/ | 459.3829[M-H-Glc]- | 2.70 | PPD |
| 667.4439[M+HOOCH-H]- | |||||||
| M5 | Rh2 | 36.002 | 667.4421 | 621.4373[M-H]-/ | 459.3814[M-H-Glc]- | 1.65 | PPD |
| 667.4432[M+HOOCH-H]- | |||||||
| M6 | PPD | 40.201 | 505.3893 | 459.3878[M-H]-/ | 221.0679[M-CHO-Glc]- | 0.79 | — |
| 505.3897[M+HOOCH-H]- |
Table 1 RRLC/Q-TOF MS data of ginsenoside Rd, Rg3, F2, CK, Rh2 and PPD
| Sample | Compd. | tR/min | Mw | MS, m/z | MS/MS fragment ion, m/z | Relative error/ (ng·mL-1) | Product |
|---|---|---|---|---|---|---|---|
| M1 | Rd | 26.187 | 991.5478 | 991.5443[M+HOOCH-H]- | 783.4929[M-H-Glc]-, | 3.53 | F2, Rg3, CK, |
| 621.4354[M-H-2Glc]-, | Rh2, PPD | ||||||
| 459.3819[M-H-3Glc]- | |||||||
| M2 | F2 | 31.421 | 829.4949 | 829.4947[M+HOOCH-H]- | 621.4308[M-H-Glc]-, | 0.24 | CK, PPD |
| 459.3821[M-H-2Glc]- | |||||||
| M3 | Rg3 | 32.693 | 829.4949 | 829.4884[M+HOOCH-H]- | 621.4311[M-H-Glc]-, | 7.84 | Rh2, PPD |
| 459.3824[M-H-2Glc]- | |||||||
| M4 | CK | 35.411 | 667.4421 | 621.4369[M-H]-/ | 459.3829[M-H-Glc]- | 2.70 | PPD |
| 667.4439[M+HOOCH-H]- | |||||||
| M5 | Rh2 | 36.002 | 667.4421 | 621.4373[M-H]-/ | 459.3814[M-H-Glc]- | 1.65 | PPD |
| 667.4432[M+HOOCH-H]- | |||||||
| M6 | PPD | 40.201 | 505.3893 | 459.3878[M-H]-/ | 221.0679[M-CHO-Glc]- | 0.79 | — |
| 505.3897[M+HOOCH-H]- |
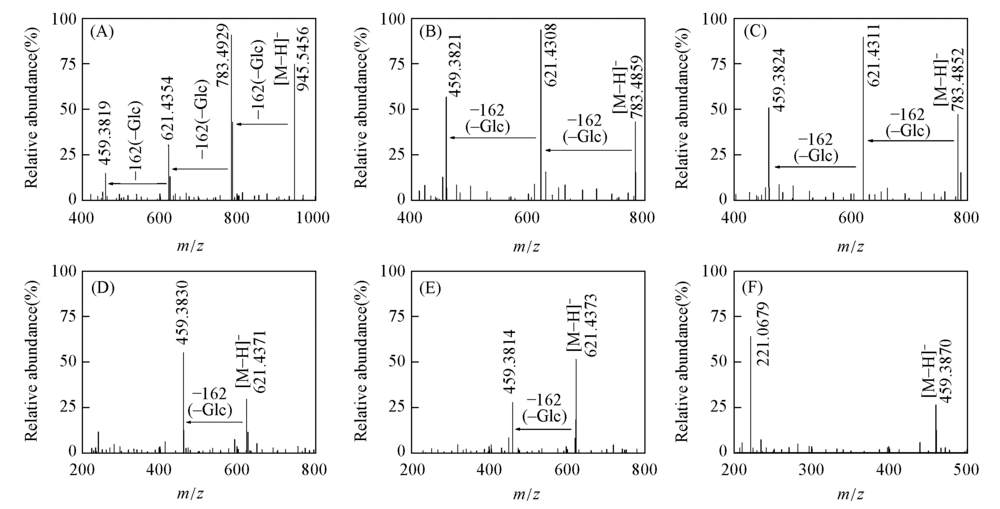
Fig.1 MS2 spectra of the metabolites of ginsenoside Rd(A), F2(B), Rg3(C), CK(D), Rh2(E) and PPD(F) under human intestinal microflora condition in vitro
| Ginsenoside | Quantitation transition | Linear regression equation | Correlation coefficient(r2) | Linear range/ (μmol·mL-1) | LOD/ (μmol·mL-1) | LOQ/ (μmol·mL-1) |
|---|---|---|---|---|---|---|
| Rd | 991.5457, 783.4927 | y=156278.26x+17382.41 | 0.995 | 0.58—142.82 | 0.032 | 0.093 |
| F2 | 829.4947, 621.4297 | y=195432.18x+32126.16 | 0.991 | 0.62—81.51 | 0.009 | 0.034 |
| Rg3 | 829.4932, 621.4307 | y=176368.23x+37525.19 | 0.992 | 0.32—72.89 | 0.007 | 0.032 |
| CK | 667.4435, 459.3828 | y=374215.12x+24656.32 | 0.998 | 0.72—186.14 | 0.033 | 0.094 |
| Rh2 | 667.4371, 459.3842 | y=283754.67x+26396.29 | 0.997 | 0.52—168.37 | 0.031 | 0.091 |
| PPD | 505.3887, 221.0676 | y=127941.39x+41259.15 | 0.994 | 0.19—192.33 | 0.035 | 0.042 |
Table 2 Calibration curves and linear range of ginsenoside Rd, F2, Rg3, CK, Rh2 and PPD
| Ginsenoside | Quantitation transition | Linear regression equation | Correlation coefficient(r2) | Linear range/ (μmol·mL-1) | LOD/ (μmol·mL-1) | LOQ/ (μmol·mL-1) |
|---|---|---|---|---|---|---|
| Rd | 991.5457, 783.4927 | y=156278.26x+17382.41 | 0.995 | 0.58—142.82 | 0.032 | 0.093 |
| F2 | 829.4947, 621.4297 | y=195432.18x+32126.16 | 0.991 | 0.62—81.51 | 0.009 | 0.034 |
| Rg3 | 829.4932, 621.4307 | y=176368.23x+37525.19 | 0.992 | 0.32—72.89 | 0.007 | 0.032 |
| CK | 667.4435, 459.3828 | y=374215.12x+24656.32 | 0.998 | 0.72—186.14 | 0.033 | 0.094 |
| Rh2 | 667.4371, 459.3842 | y=283754.67x+26396.29 | 0.997 | 0.52—168.37 | 0.031 | 0.091 |
| PPD | 505.3887, 221.0676 | y=127941.39x+41259.15 | 0.994 | 0.19—192.33 | 0.035 | 0.042 |
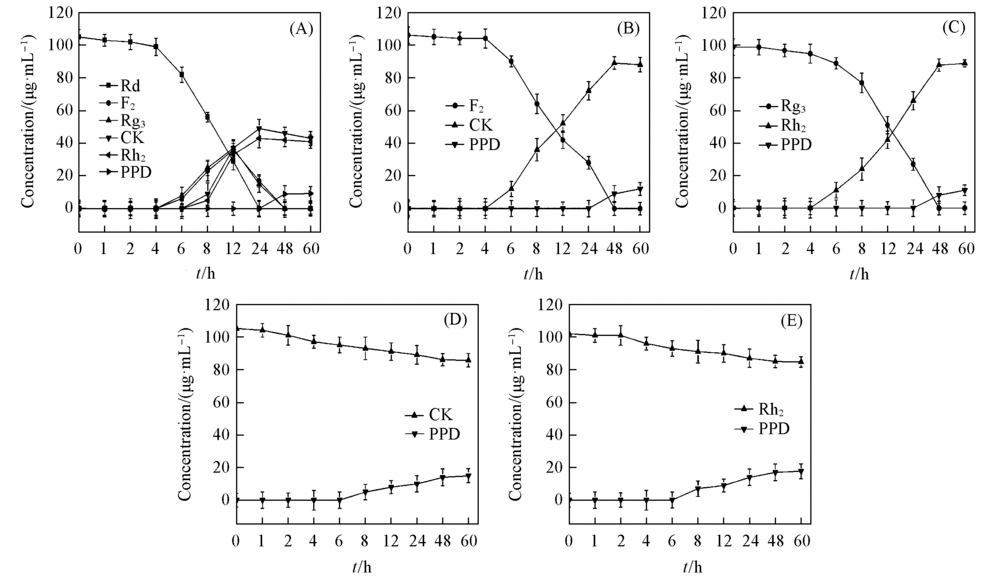
Fig.2 Time-concentration curves of ginsenoside Rd(A), F2(B), Rg3(C), CK(D), Rh2(E) and their metabolites by human intestinal microflora condition in vitro
| [1] | Dai Y. L., Yue H.,Sun C. J., Guo Y. L., Zheng F., Li J., Liu S. Y., Chinese J. Anal. Chem., 2015, 43(8), 1181—1186 |
| (戴雨霖, 越皓, 孙长江, 郭云龙, 郑飞, 李晶, 刘淑莹. 分析化学, 2015, 43(8), 1181—1186) | |
| [2] | Luo R. J., Chai W. H., Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai, 2013, 27(6), 99—102 |
| (罗瑞静, 柴维汉. 上海中医药大学学报, 2013, 27(6), 99—102) | |
| [3] | Zhang L.N., Ming Y. S., Qu B. Q., Quan Y. L., Cong J. X., Wu X. H., Chinese Brewing, 2017, 36(11), 114—117 |
| (张丽娜, 明有山, 曲波权, 全艳玲, 丛景香, 吴秀红. 中国酿造, 2017, 36(11), 114—117) | |
| [4] | Liu L. W., Si L.,Ren S. Y., Liu Y. H., Nat. Prod. Res. Dev., 2012, 24(10), 1437—1440 |
| (刘伶文, 司磊, 任树勇, 刘永红. 天然产物研究与开发, 2012, 24(10), 1437—1440) | |
| [5] | Baek S.H., Bae O. N., Park J. H., J. Ginseng Res., 2012, 36(2), 119—134 |
| [6] | Zhou Q. L., Xu W.,Yang X. W., China J. Chinese Mater. Med., 2016, 41(2), 233—249 |
| (周琪乐, 徐嵬, 杨秀伟. 中国中药杂志, 2016, 41(2), 233—249) | |
| [7] | Huang Y. T., Xu Y. S., Fan G. W., Chinese J. Clin. Pharmacol., 2017, 33(22), 2311—2313| |
| (黄鈺婷, 徐赟晟, 樊官伟. 中国临床药理学杂志, 2017, 33(22), 2311—2313) | |
| [8] | Li R., Zhong Y. G., Qian K., Pract. Pharm. Clin. Remed., 2014, 17(12), 1516—1522 |
| (李蓉, 钟益刚, 钱康. 实用药物与临床, 2014, 17(12), 1516—1522) | |
| [9] | Liu Y., Li X.W., Zhang H. Q., Wu Q., Shi X. L., Jin Y. R., Chem. Res. Chinese Universities, 2018, 34(3), 382—388 |
| [10] | Zheng F., Wang W., Yu S. S., Dai Y. L., Liu S., Wen L. K., Yue H., Chinese. J. Appl. Chem., 2017, 34(06), 723—728 |
| (郑飞, 王微, 于珊珊, 戴雨霖, 刘尚, 文连奎, 越皓. 应用化学, 2017, 34(06), 723—728) | |
| [11] | Guo Y.L., Qian J., Di L. Q., Kang A., Chinese Tradit. Herb. Drugs, 2016, 47(23), 4198—4203 |
| (郭跃龙, 钱静, 狄留庆, 康安. 中草药, 2016, 47(23), 4198—4203) | |
| [12] | Kang A., Zhang S.J., Shan J. J., Di L. Q., J. China Pharm. University, 2016, 47(2), 182—187 |
| (康安, 张圣洁, 单进军, 狄留庆. 中国药科大学学报, 2016, 47(2), 182—187) | |
| [13] | Zhang X., Wang L. N., Song F. R.,Liu Z. Q., Liu S. Y., Chinese J. Anal. Chem., 2007, 35(4), 559—563 |
| (张旭, 王丽娜, 宋凤瑞, 刘志强, 刘淑莹. 分析化学, 2007, 35(4), 559—563) | |
| [14] | Tawab M. A., Bahr U., Karas M., Wurglics M.,Schubert-Zsilavecz M., Drug Metab. Dispos., 2003, 31(8), 1065—1071 |
| [15] | Bae E. A., Park S.,Kim D. H., Biol. Pharm. Bull., 2000, 23(12), 1481—1485 |
| [16] | Qi L, W., Wang H. Y., Zhang H., Wang C. Z., Li P., Yuan C. S., J. Chromatogr. A, 2012, 1230, 93—99 |
| [17] | Ludwig A.I., Pena M. P., Cid C., BioFactors, 2013, 39(6), 623—632 |
| [18] | Yang X. W., Xu W., China J. Chinese Mater. Med., 2011, 36(1), 19—26 |
| (杨秀伟, 徐嵬. 中国中药杂志, 2011, 36(1), 19—26) | |
| [19] | Pang L. Q., Liang Q. L.,Liu Q. F., Ran X. R., Wang Y. M., Luo G. A., Chinese J. Anal. Chem., 2007, 35(10), 1421—1424 |
| (庞丽琼, 梁琼麟, 刘清飞, 冉小蓉, 王义明, 罗国安. 分析化学, 2007, 35(10), 1421—1424) | |
| [20] | Dai Y.L., Zhang Y., Zhao X., Jeon Y. J., Zheng F., Ma L., Yue H., Chem. Res. Chinese Universities, 2018, 34(3), 375—281 |
| [1] | LUO Lei, MU Xiaoqing, WU Tao, NIE Yao, XU Yan. Synthesis of Norephedrine in One Pot [J]. Chem. J. Chinese Universities, 2021, 42(8): 2458. |
| [2] | ZHU Ling,WANG Yuchen,ZHAO Jiangyuan,WEN Mengliang,LI Minggang,HAN Xiulin. Transformation of Ginsenoside Rb3 and C-Mx by Recombinant β-Xylosidase † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1010. |
| [3] | WANG Tianqi,YU Qiongwei,FENG Yuqi. Analysis of Imidazole Propionic Acid in Serum of Patients with Type 2 Diabetes Based on NiO@SiO2 Solid-phase Extraction Coupled with Liquid Chromatography-Mass Spectrometry † [J]. Chem. J. Chinese Universities, 2020, 41(2): 262. |
| [4] | WANG Lianping,LI Qingjie,LIU Xiaoyan,REN Yueying,YANG Xiuwei. Screening of Cholinesterase Inhibitors in Fructus Evodiae Alkaloids Based on UFLC-MS/molecular Simulation † [J]. Chem. J. Chinese Universities, 2020, 41(1): 111. |
| [5] | DU Chenhui, LI Ze, CUI Xiaofang, ZHANG Min, PEI Xiangping, ZHAN Haixian, YAN Yan. Chemical Transformation of Raw and Fermented Ziziphi Spinosae Semen Analyzed by UPLC-Q-Orbitrap MS/MS [J]. Chem. J. Chinese Universities, 2019, 40(8): 1614. |
| [6] |
XIAO Yongkun,LIU Chunying,YU Hongshan,YI Tea-Hoo,XU Longquan,SONG Jianguo,IM Wan-Teak,SUN Changkai,JIN Fengxie.
Dynamic Biotransformation of Protopanaxadiol-ginsenosides and Preparation of Minor Ginsenosides C-K or |
| [7] | ZHAO Huanxi,WANG Qiuying,SUN Xiuli,LI Xue,MIAO Rui,WU Dongxue,LIU Shuying,XIU Yang. Discrimination of Ginseng Origins and Identification of Ginsenoside Markers Based on HPLC-MS Combined with Multivariate Statistical Analysis† [J]. Chem. J. Chinese Universities, 2019, 40(2): 246. |
| [8] | QIAO Mengdan, LIU Shang, ZHANG Yan, LI Jing, ZHENG Fei, DAI Yulin, YUE Hao. Biotransformation of Ginsenosides in Fermented Ginseng Using UPLC-Q-Orbitrap MS/MS† [J]. Chem. J. Chinese Universities, 2018, 39(2): 219. |
| [9] | LIU Xinru, LIU Chunying, XU Longquan, SONG Jianguo, YU Hongshan. Construction of Ginsenoside-β-glucosidase Gene Vector and Biotransformation in Pichia Pastoris† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2451. |
| [10] | MIAO Rui,WU Dongxue,WANG Qiuying,ZHAO Huanxi,LI Xue,XIU Yang,LIU Shuying. Rapid Separation of Ginsenosides Based on Multi-walled Carbon Nanotubes† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2178. |
| [11] | LI Ruigang,ZHU Na,ZHAO Huanxi,WANG Nan,SUN Hongmei,YUE Hao,LI Jing. Effects of Ginseng Polysaccharides on the Metabolism of Ginsenoside Re in vivo and Transformation of Ginsenoside Re in vitro† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2192. |
| [12] | LI Nan, ZHAO Huanxi, LI Jing, WANG Nan, YU Bohao, YUE Hao, YU Shanshan. Transformation of Total Notoginsenosides by Recombinant Endocellulase Fpendo5A† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2185. |
| [13] | XU Chunchun, YU Bohao, WANG Honglei, LI Jing, LIU Shuying, YU Shanshan. Transformation of Minor Ginsenoside Rd and CK by Recombinant Thermostable β-Glucosidase† [J]. Chem. J. Chinese Universities, 2016, 37(2): 281. |
| [14] | NIU Jun, PI Zi-Feng, YUE Hao, LIU Shu-Ying. Urinary Metabonomics Study of Type 2 Diabetic Rats Treated by Glimepiride [J]. Chem. J. Chinese Universities, 2012, 33(10): 2169. |
| [15] | ZHANG Yu-Feng, FAN Xiao-Hui, QU Hai-Bin*. Novel Approach for Quality Evaluation of Traditional Chinese Medicine Based on Integrated Selected-ion Chromatograms [J]. Chem. J. Chinese Universities, 2011, 32(11): 2515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||