

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (6): 1324.doi: 10.7503/cjcu20180781
• Polymer Chemistry • Previous Articles Next Articles
SHAO Lu1, LIANG Chunchao1, XU Sheng1( ), MI Puke1(
), MI Puke1( ), WANG Tinglan1, YI Jianjun2
), WANG Tinglan1, YI Jianjun2
Received:2018-11-21
Online:2019-06-10
Published:2019-04-08
Supported by:CLC Number:
TrendMD:
SHAO Lu,LIANG Chunchao,XU Sheng,MI Puke,WANG Tinglan,YI Jianjun. Synthesis of Methylene-bridged Binuclear Metallocene Complexes and Application for Propylene Syndiospecific Polymerization†[J]. Chem. J. Chinese Universities, 2019, 40(6): 1324.
| Catalyst | Activity/[106g polymer/(molM·h)] | 10-4 Mn | 10-5 Mw | MWD |
|---|---|---|---|---|
| 4(FZr2) | 3.2 | 8.36 | 4.88 | 5.84 |
| 8(FZr1) | 2.5 | 7.32 | 1.23 | 1.68 |
| 5(FTi2) | 1.7 | 10.90 | 5.42 | 4.97 |
| 9(FTi1) | 1.3 | 5.80 | 1.09 | 1.88 |
| 6(IZr2) | 7.5 | 10.00 | 5.57 | 5.56 |
| 10(IZr1) | 2.1 | 14.70 | 2.52 | 1.71 |
| 7(ITi2) | 1.2 | 13.80 | 7.51 | 5.43 |
| 11(ITi1) | 0.8 | 26.40 | 5.05 | 1.91 |
Table 1 Results of ethylene polymerization with complexes 1—11*
| Catalyst | Activity/[106g polymer/(molM·h)] | 10-4 Mn | 10-5 Mw | MWD |
|---|---|---|---|---|
| 4(FZr2) | 3.2 | 8.36 | 4.88 | 5.84 |
| 8(FZr1) | 2.5 | 7.32 | 1.23 | 1.68 |
| 5(FTi2) | 1.7 | 10.90 | 5.42 | 4.97 |
| 9(FTi1) | 1.3 | 5.80 | 1.09 | 1.88 |
| 6(IZr2) | 7.5 | 10.00 | 5.57 | 5.56 |
| 10(IZr1) | 2.1 | 14.70 | 2.52 | 1.71 |
| 7(ITi2) | 1.2 | 13.80 | 7.51 | 5.43 |
| 11(ITi1) | 0.8 | 26.40 | 5.05 | 1.91 |
| Entry | Catalyst | Activity/[105 g polymer/(mol Zr·h)] | 10-4 | 10-4 | MWD | r(%) |
|---|---|---|---|---|---|---|
| 1 | 4(FZr2) | 10.0 | 13.0 | 3.1 | 90 | |
| 2 | 8(FZr1) | 8.0 | 5.2 | 1.8 | 92 | |
| 3 | 5(FTi2) | 2.8 | 16.0 | 70 | ||
| 4 | 9(FTi1) | 1.9 | 12.5 | 76 | ||
| 5 | 6(IZr2) | 12.0 | 10.0 | 2.9 | 45 | |
| 6 | 10(IZr1) | 8.5 | 4.5 | 1.8 | 56 | |
| 7 | 7(ITi2) | 4.5 | 12.0 | 30 | ||
| 8 | 11(ITi1) | 3.2 | 9.7 | 40 |
Table 2 Polymerization results of propylene with catalysts 4—11a
| Entry | Catalyst | Activity/[105 g polymer/(mol Zr·h)] | 10-4 | 10-4 | MWD | r(%) |
|---|---|---|---|---|---|---|
| 1 | 4(FZr2) | 10.0 | 13.0 | 3.1 | 90 | |
| 2 | 8(FZr1) | 8.0 | 5.2 | 1.8 | 92 | |
| 3 | 5(FTi2) | 2.8 | 16.0 | 70 | ||
| 4 | 9(FTi1) | 1.9 | 12.5 | 76 | ||
| 5 | 6(IZr2) | 12.0 | 10.0 | 2.9 | 45 | |
| 6 | 10(IZr1) | 8.5 | 4.5 | 1.8 | 56 | |
| 7 | 7(ITi2) | 4.5 | 12.0 | 30 | ||
| 8 | 11(ITi1) | 3.2 | 9.7 | 40 |
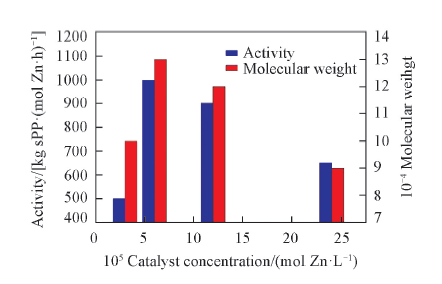
Fig.4 Influence of catalyst concentrstion on polymerization activity and molecular weight Polymerization conditions: n(Al)/n(Zr)=2000, 0.5 h, 60 ℃, 0.4 MPa, V(toluene)=100 mL.
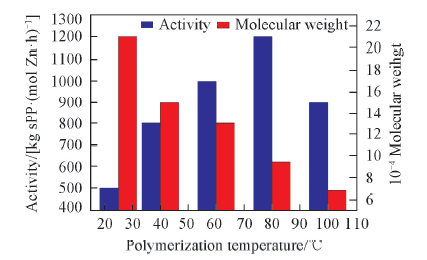
Fig.5 Influence of polymerization temperature on polymerization activity and molecular weight Polymerization conditions: 1×10-5 mol Zr/L, n(Al)/n(Zr)=2000, 0.5 h, 0.4 MPa, V(toluene)=100 mL.
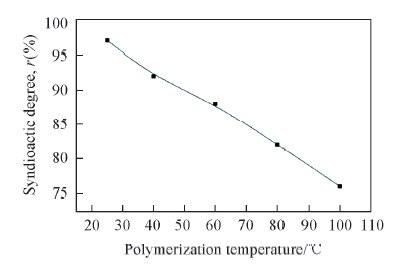
Fig.6 Influence of polymerization temperature on polymer syndiotactic degree Polymerization conditions: 1×10-5 mol Zr/L, n(Al)/n(Zr)=2000, 0.5 h, 0.4 MPa, V(toluene)=100 mL.
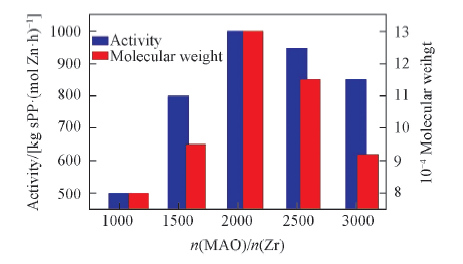
Fig.7 Influence of n(MAO)/n(Zr) on polymerization activity and polymer molecular weight Polymerization conditions: 1×10-5 mol Zr/L, 60 ℃, 0.5 h, 0.4 MPa, V(toluene)=100 mL.
| [1] | Hao P., Zhang S., Yi J. J., Sun W. H., J. Mol. Catal. A: Chem.,2009, 302, 1-6 |
| [2] | Delferro M., Marks T. J., Chem. Rev.,2011, 111, 2450-2485 |
| [3] | Motta A.,FragalãI. L.,Marks T. J. J. Am. Chem. Soc.,2009, 131, 3974-3984 |
| [4] | Suo H. Y., Solan G. A., Ma Y. P., Sun W. H., Coord. Chem. Rev.,2018, 372, 101-116 |
| [5] | Ewen J. A., J. Am. Chem. Soc., 1984, 106(21), 6355-6364 |
| [6] | Zhang Y. T., Ning Y. L., Caporaso L., Cavallo L., Chen E. Y. X., J. Am. Chem. Soc.,2010, 132, 2695-2709 |
| [7] | Lin W. F., Tsai J. C., J. Polym. Sci., Part A: Polym. Chem.,2008, 46(6), 2167-2176 |
| [8] | Lin W. F., Hsiao T. J., Tsai J. C., Chung T. M., Ho R. M., J. Polym. Sci., Part A: Polym. Chem.,2008, 46(14), 4843-4856 |
| [9] | Kaminsky W., Strübel C., Lechert H., Genske D., Woo S. I., Macromol. Rapid Commun.,2000, 21, 909-912 |
| [10] | Herzog T. A., Zubris D. L., Bercaw J. E., J. Am. Chem. Soc., 1996, 118, 11988-11989 |
| [11] | Saito J. J., Mitan M., Onda M., Mohri J. I., Ishii S. I., Yoshida Y., Nakano T., Tanaka H., Matsugi T., Kojoh S. I., Kashiwa N., Fujita T., Macromol. Rapid Commun.,2001, 22, 1072-1075 |
| [12] | Miyake G. M., Chen E. Y. X., Polym. Chem., 2011, 2, 2462-2480 |
| [13] | Stephan J., Rolf M., Herbert P., J. Organomet. Chem.,1993, 460(2), 191-195 |
| [14] | Green M. L. H., Ishihara N., J. Chem. Soc. Dalton Trans.,1994, 5(5), 657-665 |
| [15] | Diamond G.M., Chernega A. N., Mountford P., Green M. L. H.,J. Chem. Soc. Dalton Trans., 1996, 921-938 |
| [16] | Yan X. F., Chernega A., Green M. L. H., Sanders J., Souter J., Ushioda T., J. Mol. Catal. A: Chem.,1998, 128, 119-141 |
| [17] | Wu Q. L., Su Q., Ye L., Li G. H., Mu Y., Dalton Trans.,2010, 39, 2525-2535 |
| [18] | Sun T. X., Yi J. J., Wang Y. G., Zhang M. G., Yuan Y., Mao J., Binuclear Heterocyclic Catalyst and Application Thereof in Propylene Homopolymerization and Copolymerization CN106543302A,2015-09-18 |
| (孙天旭, 义建军, 王永刚, 张明革, 袁苑, 毛静. 双核杂环催化剂及其在丙烯均聚和共聚中的应用, CN106543302A, 2015-09-18) | |
| [19] | Xu S., Huang J. L., J. Appl. Polym. Sci.,2013, 130(4), 2891-2900 |
| [20] | Khalil C., Henrik O., Cheminform,2004, 45, 6741-6744 |
| [21] | Erickson M. S., Cronan J. M., Garcia J. G., J. Org. Chem.,1992, 57(8), 2504-2508 |
| [22] | Razavi A., Atwood J. L., J. Organomet. Chem.,1996, 520(s1/2), 115-120 |
| [23] | Wang B. Q., Mu B., Deng X. B., Cui H. L., Xu S. S., Zhou X. Z., Zou F. L., Li Y., Yang L., Yi Y. F., Ha Y. L., Chem. A: Eur. J.,2004, 11(2), 669-679 |
| [24] | Li T. C., Lan Z., Xie G. Y., Luo D. R., Li L., Xiong S. F., Zhang L., Ou Y., Li P., Zhang A. Q., Catal. Lett.,2017, 147(4), 996-1005 |
| [25] | Li W. W.,Mu H. L.Liu J. Y., Li Y. S., J. Organomet. Chem.,2017, 836/837, 34-43 |
| [26] | Chen Z. T., Yao E. D., Wang J. C., Gong X. Y., Ma Y. G., Macromolecules,2016, 49, 8848-8854 |
| [27] | Chen Z. T., Zhao X. X., Gong X. Y., Xu D., Ma Y. G., Macromolecules,2017, 50, 6561-6568 |
| [28] | Xu D., Zhao X. X., Chen Z. T., Ma Y. G., Chinese J. Polym. Sci.,2018, 36(2), 244-251 |
| [29] | Xing Q. F., Song K. F., Liang T. L., Liu Q. B., Sun W. H., Redshaw C., Dalton Trans.,2014, 43, 7830-7837 |
| [30] | Luo G., Luo Y., Hou Z. M., Qu J. P., Organometallics,2016, 35, 778-784 |
| [31] | Ye J. D., Ye Z. B., Polymers,2017, 9(282), 1-21 |
| [32] | Brintzinger H. H., Fischer D., Mülhaupt R., Angew. Chem. Int. Ed.,1995, 34(11), 1143-1170 |
| [1] | JIAO Long, DAI Xuemin, MU Jianxin, DU Zhijun, WANG Hanfu, DONG Zhixin, QIU Xuepeng. Preparation and Properties of High Heat-resistant Polyimide Films for Flexible OLED [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220390. |
| [2] | XU Huan, KE Lyu, TANG Mengke, SHANG Han, XU Wenxuan, ZHANG Zilin, FU Yanan, HAN Guangdong, CUI Jinsheng, YANG Haoran, GAO Jiefeng, ZHANG Shenghui, HE Xinjian. In⁃situ Liquid Exfoliation of Montmorillonite Nanosheets in Poly(lactic acid) to Resist Oxygen Permeation [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220316. |
| [3] | YANG Weiming, XI Aoqian, YANG Bin, ZENG Yanning. Fabrication and Properties of Epoxy Vitrimer Based on Multiply Dynamic Covalent Bonds [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220308. |
| [4] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [5] | ZHANG Tao, SHAO Liang, ZHANG Menghui, MA Zhonglei, LI Xiaoqiang, MA Jianzhong. Preparation and Properties of Bifunctional Polydimethysiloxane/Copper Nanowire Composite Films [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220359. |
| [6] | YAN Shuting, YAO Yuan, TAO Xinfeng, LIN Shaoliang. Synthesis and Properties of Polypeptoid Hydrogels Containing Sulfonium Groups [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220381. |
| [7] | TAN Lejian, ZHONG Xuanshu, WANG Jin, LIU Zongjian, ZHANG Aiying, YE Lin, FENG Zengguo. Low Critical Dissolution Temperature Behavior of β⁃Cyclodextrin and Its Application in the Preparation of β⁃Cyclodextrin Sheet Crystal with Ordered Nano⁃channel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220405. |
| [8] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [9] | WANG Shoubai, WU Xiuming, SHU Chen, ZHONG Min, HUANG Wei, YAN Deyue. Gas Separation Performance of Polyimide Homogeneous MembranesContaining tert-Butyl Groups [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220357. |
| [10] | JIA Hongjun, ZHANG Jiatao, MA Zhuoli, WANG Heng, YANG Xinyu, YANG Jiazhi. Preparation of PTFE/PAA/Nafion Composite Membrane by Aqueous Polymerization of Acrylic Acid and Its Properties [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220350. |
| [11] | ZHU Ge, LI Zhihan, LIU Kun, SUN Tianmeng. Effect of Aluminium Nanopowders on Activation of Dendritic Cells [J]. Chem. J. Chinese Universities, 0, (): 20220602. |
| [12] | LI Jichen, CAI Shanshan, PENG Jubo, LI Hongfei, DUAN Xiaozheng. Structural Variations of Ionic Polymeric Vesicles under Electric Field: A Molecular Dynamics Simulation Study [J]. Chem. J. Chinese Universities, 0, (): 20220553. |
| [13] | . Novel Biodegradable Nanodrugs ZnO2@Fe3+-TA@PVP for Chemodynamic Therapy [J]. Chem. J. Chinese Universities, 0, (): 20220554. |
| [14] | LI Lun, ZHANG Jingyan, LUO Jing, LIU Ren, ZHU Yi. Synthesis and Properties of UV/Vis-LED Excitable Photoinitiators Based on Coumarin Pyridinium Salt [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220178. |
| [15] | XU Wenzhe, ZHANG Hao. Supramolecular Interactions-mediated Nanodrug Nucleation [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220264. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||