

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (4): 698.doi: 10.7503/cjcu20180698
• Organic Chemistry • Previous Articles Next Articles
FENG Xian1,2, MU Xiaoqing1,2,*( ), NIE Yao1, XU Yan1
), NIE Yao1, XU Yan1
Received:2018-10-16
Online:2019-01-24
Published:2019-01-24
Contact:
MU Xiaoqing
E-mail:xqmu@jiangnan.edu.cn
Supported by:CLC Number:
TrendMD:
FENG Xian,MU Xiaoqing,NIE Yao,XU Yan. Synthesis of α-Ketoisocaproate Through Substrate Coupling Reaction Catalyzed by Leucine Dehydrogenase†[J]. Chem. J. Chinese Universities, 2019, 40(4): 698.
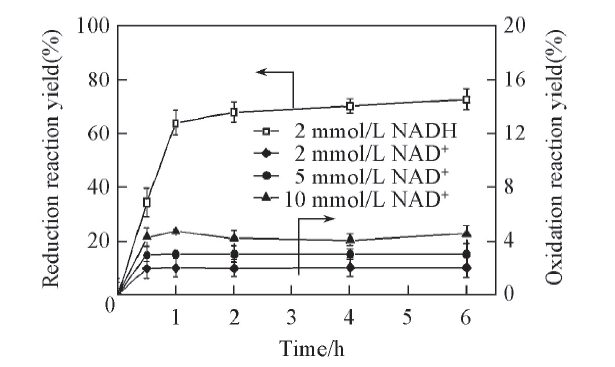
Fig.1 Time curves of oxidation of L-leucine and reduction of α-KIC catalyzed by LeuDHAll reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5). c(α-KIC)=10 mmol/L, c(L-Leucine)=10 mmol/L.
| Substrate | Structural formula | Specific activity/ (U·mg-1) | Vm/ (mol·min-1·mg-1) | Km/ (mmol·L-1) | (kcat/Km)/ (L·mmol-1·s-1) |
|---|---|---|---|---|---|
| α-KIC | | 11.14 | 695.4 | 2.38 | 26.14 |
| L-Leucine | | 1.66 | 2.75 | 24.56 | 0.012 |
Table 1 Enzymatic oxidation and reduction properties of LeuDH for L-leucine and α-KIC
| Substrate | Structural formula | Specific activity/ (U·mg-1) | Vm/ (mol·min-1·mg-1) | Km/ (mmol·L-1) | (kcat/Km)/ (L·mmol-1·s-1) |
|---|---|---|---|---|---|
| α-KIC | | 11.14 | 695.4 | 2.38 | 26.14 |
| L-Leucine | | 1.66 | 2.75 | 24.56 | 0.012 |
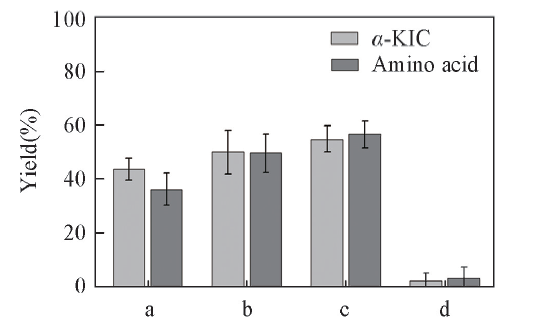
Fig.2 Effects of coupling substrates to the yield of α-KICAll reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising amino acid(10 mmol/L), ketonic acid(10 mmol/L) and NAD+(0.2 mmol/L). a. Trimethylpyruvic acid; b. 3-methyl-2-oxopentanoic acid; c. 2-oxobutyrate; d. pyruvic acid.
| Substrate | Structural formula | Specific activity /(U·mg-1) | (kcat/Km)/(L·mmol-1·s-1) | Yield(%) | ||
|---|---|---|---|---|---|---|
| 2-Oxobutyrate | | 21.32 | 64.22 | 73.73 | ||
| L-2-aminobutyrate | | 0.61 | 0.01 | 1.14 | ||
| α-KIC | | 52.77 | 143.69 | 66.82 | ||
| L-valine | | 0.71 | 0.03 | 2.94 | ||
| α-Ketoisocaproate | | 11.14 | 26.14 | 70.36 | ||
| L-leucine | | 1.66 | 0.01 | 2.09 | ||
| Trimethylpyruvic acid | | 7.99 | 1.84 | 65.46 | ||
| L-tert-leucine | | —— | —— | —— | ||
Table 2 Enzymatic oxidation and reduction properties of LeuDH for different kinds of amino acids and ketonic acids
| Substrate | Structural formula | Specific activity /(U·mg-1) | (kcat/Km)/(L·mmol-1·s-1) | Yield(%) | ||
|---|---|---|---|---|---|---|
| 2-Oxobutyrate | | 21.32 | 64.22 | 73.73 | ||
| L-2-aminobutyrate | | 0.61 | 0.01 | 1.14 | ||
| α-KIC | | 52.77 | 143.69 | 66.82 | ||
| L-valine | | 0.71 | 0.03 | 2.94 | ||
| α-Ketoisocaproate | | 11.14 | 26.14 | 70.36 | ||
| L-leucine | | 1.66 | 0.01 | 2.09 | ||
| Trimethylpyruvic acid | | 7.99 | 1.84 | 65.46 | ||
| L-tert-leucine | | —— | —— | —— | ||
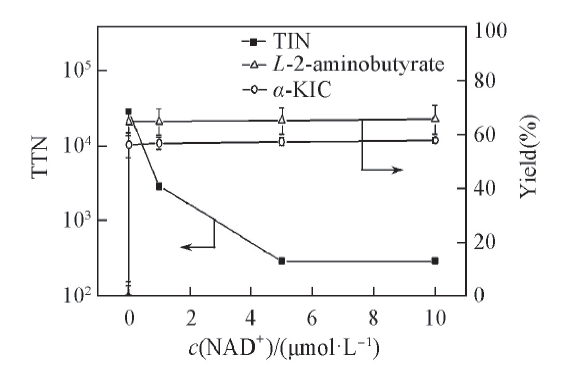
Fig.3 Effects of initial NAD+ concentration on TTN and the yield of α-KICAll reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising L-leucine(10 mmol/L), 2-oxobutyrate(10 mmol/L) and NAD+.
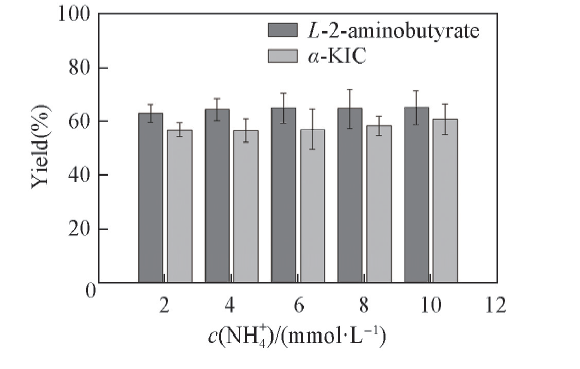
Fig.4 Effects of NH4+ concentration on the yield of α-KIC All reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising L-leucine(10 mmol/L), 2-oxobutyrate(10 mmol/L) and NAD+(0.01 μmol/L).
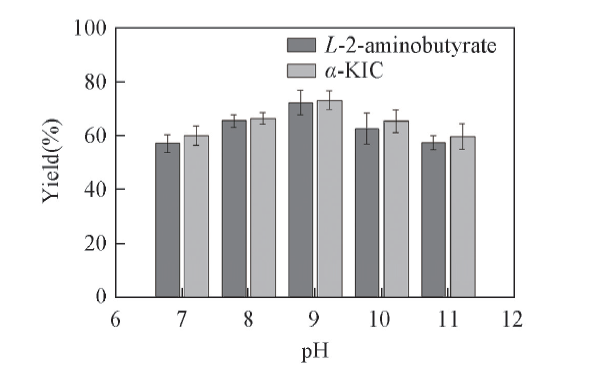
Fig.5 Effects of pH value on the yield of α-KIC All reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising L-leucine(10 mmol/L), 2-oxobutyrate(10 mmol/L) and NAD+(0.01 μmol/L).
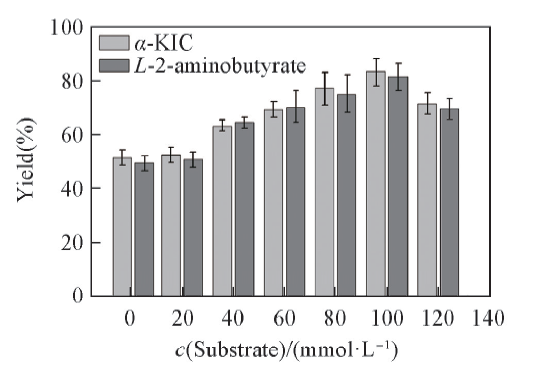
Fig.6 Effects of substrate concentration on the yield of α-KIC All reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising L-leucine, 2-oxobutyrate and NAD+(0.01 μmol/L).
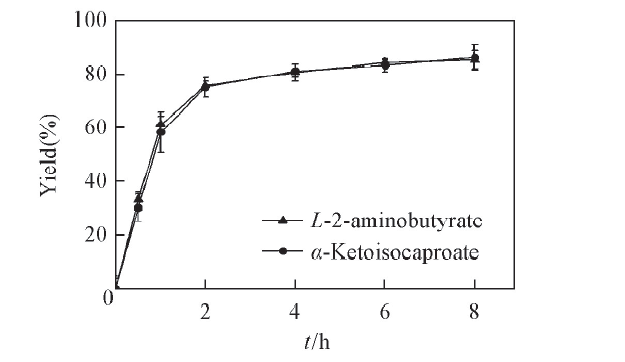
Fig.7 Time curves of the substrate coupling reaction systemAll reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising L-leucine(100 mmol/L), 2-oxobutyrate(100 mmol/L) and NAD+ (0.01 μmol/L).
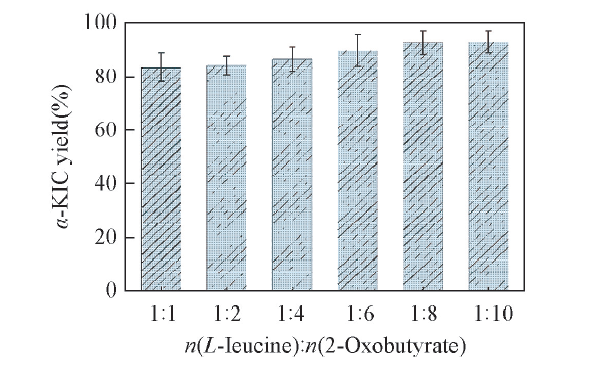
Fig.8 Effects of the molar ratios of L-leucine and 2-oxobutyrate on the yield of α-KICAll reactions were carried out in 2 mL Tris-HCl buffer(0.1 mol/L, pH=8.5) comprising L-leucine(10 mmol/L), 2-oxobutyrate and NAD+(0.01 μmol/L).
| [1] | Zhou Y. S., Jetton T. L., Goshorn S., Lynch C. J., She P. X., J. Biol.Chem.,2010, 285(44), 33718—33726 |
| [2] | Yang J. C., Chi Y. J., Burkhardt B. R., Guan Y. F., Wolf B. A., Nutr.Rev.,2010, 68(5), 270—279 |
| [3] | Taschetto L., Scaini G., Zapelini H. G., Ramos Â. C., Strapazzon G., Andrade V. M., Réus G. Z., Michels M., Dal-Pizzol F., Quevedo J.,Metab. Brain Dis.,2017, 32(5), 1—12 |
| [4] | Heissig H., Urban K. A., Hastedt K., Zünkler B. J., Panten U., Mol.Pharmacol.,2005, 68(4), 1097—1105 |
| [5] | Wisniewski M. S. W., Carvalho-Silva M., Gomes L. M., Zapelini H. G., Schuck P. F., Ferreira G. C., Scaini G., Streck E. L., Metab. Brain Dis.,2016, 31(2), 377—383 |
| [6] | Girón M. D., Vílchez J. D., Rafael S., Manuel M., Natalia S., Nefertiti C., Argilés J. M., Ricardo R., López-Pedrosa J. M., J. Cachexia Sarcopeni.,2016, 7(1), 68—78 |
| [7] | van Someren K. A., Edwards A. J., Howatson G., Int. J. Sport Nutr. Exerc. Metab.,2005, 15(4), 413—424 |
| [8] | Escobar J., Frank J. W., Suryawan A., Nguyen H. V., van Horn C. G., Hutson S. M., Davis T. A., J.Nutr.,2010, 140(8), 1418—1424 |
| [9] | Ogo S., Uehara K., Abura T., Fukuzumi S., J. Am. Chem.Soc.,2004, 126(10), 3020—3021 |
| [10] | Hidalgo F. J., Delgado R. M., Zamora R.,Food Chem.,2013, 141(2), 1140—1146 |
| [11] | Aparicio M., Bellizzi V., Chauveau P., Cupisti A., Ecder T., Fouque D., Garneata L., Lin S., Mitch W. E., Teplan V., J. Ren.Nutr.,2012, 22(2), S1—S21 |
| [12] | Mou S., Li J., Yu Z., Wang Q., Ni Z., J. Int. Med. Res. ,2013, 41(1), 129—137 |
| [13] | Holecek M., Nutr., 2010, 26(5), 482—490 |
| [14] | Norton L. E., Wilson G. J., Layman D. K., Moulton C. J., Garlick P. J., Nutr.Metab.,2012, 9(1), 67—76 |
| [15] | Cann A. F., Liao J. C., Appl. Microbiol.Biotechnol.,2010, 85(4), 893—899 |
| [16] | Freiding S., Ehrmann M. A., Vogel R. F., Food Microbiol.,2012, 29(2), 205—214 |
| [17] | Geueke B., Hummel W., Enzyme Microb.Tech.,2002, 31(1/2), 77—87 |
| [18] | Pollegioni L., Molla G., Sacchi S., Rosini E., Verga R., Pilone M. S., Appl. Microbiol.Biotechnol.,2008, 78(1), 1—16 |
| [19] | Du X. Y., Clemetson K. J., Toxicon,2002, 40(6), 659—665 |
| [20] | Baud D., Jeffries J. W. E., Moody T. S., Ward J. M., Hailes H. C., Green Chem.,2017, 19(4), 1134—1143 |
| [21] | Jin J. Z., Chang D. L., Zhang J., Appl. Biochem.Biotechnol.,2011, 164(3), 376—385 |
| [22] | Farnberger J. E., Lorenz E., Richter N., Wendisch V. F., Kroutil W., Microb. Cell Fact.,2017, 16(1), 132—148 |
| [23] | Chen X., Gao X. Z., Zhu D. M., Acta Microbiol.Sinica,2017, 57(8), 1249—1261 |
| (陈曦, 高秀珍, 朱敦明. 微生物学报, 2017, 57(8), 1249—1261) | |
| [24] | Xue Y. P., Cao C. H., Zheng Y. G., Chem. Soc.Rev.,2018, 47(4), 1516—1561 |
| [25] | Katoh R., Ngata S., Ozawa A., Ohshima T., Kamekura M., Misono H., J. Mol. Catal. B: Enzym.,2003, 23(2—6), 231—238 |
| [26] | Zhu D. M., Hua L., J.Biotechnol.(,2009, 410), 1420—1431 |
| [27] | Miao W. J., Wang P., Zhang S. P., Chinese J. Proc.Eng.,2008, 8(1), 102—108 |
| (苗维娟, 王平, 张松平. 过程工程学报, 2008, 8(1), 102—108) | |
| [28] | Seelbach K., Riebel B., Hummel W., Kula M. R., Tishkov V. I., Egorov A. M., Wandrey C., Kragl U., Tetrahedron Lett.,1996, 37(9), 1377—1380 |
| [29] | Kara S., Spickermann D., Schrittwieser J. H., Leggewie C., Berkel W. J. H. V., Arends I. W. C. E., Hollmann F., Green Chem.,2013, 15(2), 330—335 |
| [30] | Lu J., Zhang Y., Sun D., Jiang W., Wang S., Fang B., Appl. Biochem.Biotechnol.,2016, 180(6), 1180—1195 |
| [31] | Kajiwara S., Maeda H., Agric. Biol.Chem.,2006, 51(11), 2873—2879 |
| [32] | Katoh R., Nagata S., Misono H., J. Mol. Catal. B: Enzym.,2003, 23(2—6), 239—247 |
| [33] | Akhteruzzaman S., Kato Y., Kouzuki H., Suzuki H., Hisaka A., Stieger B., Meier P. J., Sugiyama Y., Phramacol. Exp.Ther.,1999, 290(3), 1107—1115 |
| [34] | Li J., Genome Data Mining of Leucine Dehydrogenase and Its Catalytic Performance in Reductive Amination of Trimethylpyruvic Acid to L-tert-Leucine, East China University of Science and Technology, Guangzhou, 2014 |
| (李静. 亮氨酸脱氢酶的基因发掘、 催化性能及其应用研究,广州: 华南理工大学, 2014) | |
| [35] | Wa V. D. D., Zhao H., Curr. Opin.Biotechnol.,2003, 14(4), 421—426 |
| [36] | Chenault H. K., Whitesides G. M., Appl. Biochem.Biotechnol.,1987, 14(2), 147—197 |
| [37] | Sun T. Q., Li B., Nie Y., Wang D., Xu Y., Chem. J. Chinese Universities,2017, 38(10), 1772—1777 |
| (孙太强, 李斌, 聂尧, 王栋, 徐岩. 高等学校化学学报, 2017, 38(10), 1772—1777) | |
| [38] | Xu C., Yin X., Zhang C., Chen H., Huang H., Hu Y., Chem. Res. Chinese Universities,2018, 34(2), 279—284 |
| [39] | Zhang J. L., Tao S. S., Zhang B. J., Wu X. R., Chen Y. J., ACS Catal.,2014, 4(5), 1584—1587 |
| [1] | MA Yunqian, WANG Rui. H2S Absorption Capacity and Regeneration Performance of Amine Fe-based Ionic Liquid† [J]. Chem. J. Chinese Universities, 2014, 35(4): 760. |
| [2] | ZHI Hui-Zhen, WANG Ying-Lei, ZHANG Qiang, LUO Jun. Reduction of Nitroarenes in PEG1000-DIL/Toluene Thermoregulated Biphasic System [J]. Chem. J. Chinese Universities, 2013, 34(3): 573. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||