

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (4): 725.doi: 10.7503/cjcu20180599
• Organic Chemistry • Previous Articles Next Articles
ZHANG Shuo*( ), YU Yitao, LI Qinggang, ZHAO Ning, HOU Zitong, LIU Yifan, LI Bing, MU Qiuhong, LI Jinhui, WANG Feng, PENG Dan*(
), YU Yitao, LI Qinggang, ZHAO Ning, HOU Zitong, LIU Yifan, LI Bing, MU Qiuhong, LI Jinhui, WANG Feng, PENG Dan*( )
)
Received:2018-08-27
Online:2019-04-03
Published:2019-01-15
Contact:
ZHANG Shuo,PENG Dan
E-mail:e50687e@163.com;lonarpeng@aliyun.com
Supported by:CLC Number:
TrendMD:
ZHANG Shuo,YU Yitao,LI Qinggang,ZHAO Ning,HOU Zitong,LIU Yifan,LI Bing,MU Qiuhong,LI Jinhui,WANG Feng,PENG Dan. Sc(Ⅲ) Catalyzed Nucleophilic Addition of in situ Generated ortho-Quinone Methides with Thiols: an Efficient Access to ortho-Hydroxybenzyl Thioethers†[J]. Chem. J. Chinese Universities, 2019, 40(4): 725.
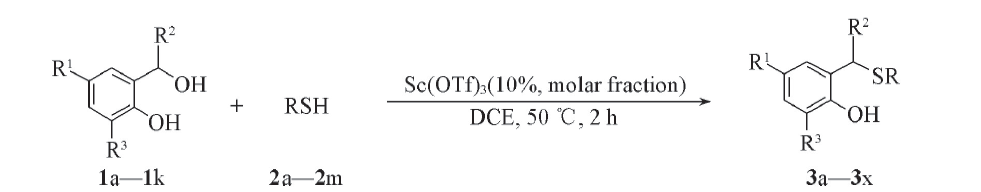
Scheme 1 Sc(OTf)3 catalyzed nucleophilic addition reaction by thiols3a: R1=H, R2=Ph, R3=H, R=C2H5; 3b: R1=H, R2=Ph, R3=H, R=n-propyl; 3c: R1=H, R2=Ph, R3=H, R=n-pentyl; 3e: R1=H, R2=Ph, R3=H, R=heptyl; 3f: R1=H, R2=Ph, R3=H, R=isobutyl; 3g: R1=H, R2=Ph, R3=H, R=isopentyl; 3h: R1=H, R2=Ph, R3=H, R=isopropyl; 3i: R1=H, R2=Ph, R3=H, R=cyclopentyl; 3j: R1=H, R2=Ph, R3=H, R=benzyl; 3k: R1=H, R2=Ph, R3=H, R=4-(tert-butyl)benzyl; 3l: R1=H, R2=Ph, R3=H, R=4-chlorobenzyl; 3m: R1=H, R2=Ph, R3=H, R=4-methylbenzyl; 3n: R1=H, R2=Ph, R3=tert-butyl, R=C2H5; 3o: R1=H, R2=Ph, R3=ethoxy, R=C2H5; 3p: R1=CH3, R2=Ph, R3=H, R=C2H5; 3q: R1=emthoxy, R2=Ph, R3=H, R=C2H5; 3r: R1=H, R2=o-tolyl, R3=H, R=C2H5; 3s: R1=H, R2=4-chlorophenyl, R3=H, R=C2H5; 3t: R1=H, R2=3-fluorophenyl, R3=H, R=C2H5; 3u: R1=H, R2=4-fluorophenyl, R3=H, R=C2H5; 3v: R1=Cl, R2=Ph, R3=H, R=C2H5; 3w: R1=H, R2=cyclohexyl, R3=H, R=C2H5; 3x: R1=H, R2=H, R3=H, R=C2H5
| Entry | Catalyst | Solvent | Temperature/℃ | t/h | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | Ga(OTf)3 | DCE | 50 | 4 | 0 |
| 2 | In(OTf)3 | DCE | 50 | 4 | 0 |
| 3 | Sc(OTf)3 | DCE | 50 | 4 | 92 |
| 4 | Conc. HCl | DCE | 50 | 8 | 56 |
| 5 | H2SO4 | DCE | 50 | 8 | 52 |
| 6 | AlCl3 | DCE | 50 | 8 | 62 |
| 7 | Sc(OTf)3 | Toluene | 50 | 4 | 72 |
| 8 | Sc(OTf)3 | THF | 50 | 4 | 76 |
| 9 | Sc(OTf)3 | DCE | 50 | 2 | 92 |
| 10 | Sc(OTf)3 | DCE | 50 | 1 | 75 |
| 11 | Sc(OTf)3 | DCE | 40 | 6 | 82 |
| 12c | Sc(OTf)3 | DCE | 50 | 6 | 78 |
| 13d | Sc(OTf)3 | DCE | 50 | 2 | 93 |
Table 1 Optimization of reaction conditionsa
| Entry | Catalyst | Solvent | Temperature/℃ | t/h | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | Ga(OTf)3 | DCE | 50 | 4 | 0 |
| 2 | In(OTf)3 | DCE | 50 | 4 | 0 |
| 3 | Sc(OTf)3 | DCE | 50 | 4 | 92 |
| 4 | Conc. HCl | DCE | 50 | 8 | 56 |
| 5 | H2SO4 | DCE | 50 | 8 | 52 |
| 6 | AlCl3 | DCE | 50 | 8 | 62 |
| 7 | Sc(OTf)3 | Toluene | 50 | 4 | 72 |
| 8 | Sc(OTf)3 | THF | 50 | 4 | 76 |
| 9 | Sc(OTf)3 | DCE | 50 | 2 | 92 |
| 10 | Sc(OTf)3 | DCE | 50 | 1 | 75 |
| 11 | Sc(OTf)3 | DCE | 40 | 6 | 82 |
| 12c | Sc(OTf)3 | DCE | 50 | 6 | 78 |
| 13d | Sc(OTf)3 | DCE | 50 | 2 | 93 |
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
|---|---|---|---|---|
| 3a | Colorless oil | 92 | 245.0996 | 3290, 3263, 3029, 1596, 1582, 1488, 1454, 1340, 1269, 1234, 754, 699 |
| 3b | Colorless oil | 90 | 259.1150 | 3289, 3062, 3029, 1596, 1582, 1487, 1454, 1339, 1270, 1236, 754, 699 |
| 3c | Colorless oil | 86 | 273.1312 | 3291, 3029, 1596, 1582, 1487, 1454, 1356, 1272, 1233, 754, 699 |
| 3d | Colorless oil | 84 | 287.1465 | 3292, 3029, 1596, 1582, 1487, 1454, 1341, 1271, 1234, 754, 699 |
| 3e | Colorless oil | 82 | 315.1779 | 3290, 3029, 1596, 1582, 1487, 1454, 1356, 1271, 1234, 753, 699 |
| 3f | Colorless oil | 87 | 273.1305 | 3291, 3029, 1597, 1583, 1488, 1454, 1366, 1271, 1234, 754, 699 |
| 3g | Colorless oil | 85 | 287.1467 | 3290, 3029, 1597, 1583, 1488, 1454, 1367, 1273, 1233, 754, 699 |
| 3h[ | Colorless oil | 88 | 259.1150 | 3290, 3029, 1598, 1583, 1489, 1454, 1366, 1271, 1235, 754, 699 |
| 3i | Colorless oil | 86 | 285.1308 | 3289, 3028, 1644, 1597, 1489, 1453, 1365, 1271, 1235, 754, 699 |
| 3j[ | Colorless oil | 91 | 307.1152 | 3289, 3028, 1644, 1599, 1493, 1454, 1341, 1272, 1234, 755, 699 |
| 3k | Colorless oil | 95 | 363.1777 | 3289, 3030, 1647, 1599, 1488, 1456, 1365, 1271, 1236, 756, 701 |
| 3l | Colorless oil | 86 | 341.0762 | 3296, 3029, 1644, 1597, 1490, 1454, 1365, 1272, 1233, 755, 699 |
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
| 3m | Colorless oil | 92 | 321.1305 | 3288, 3027, 1645, 1596, 1486, 1454, 1353, 1271, 1235, 754, 699 |
| 3n | Colorless oil | 90 | 301.1622 | 3246, 3028, 1655, 1600, 1586, 1494, 1483, 1363, 1268, 1231, 749, 701 |
| 3o | Colorless oil | 82 | 289.1257 | 3525, 3028, 1662, 1614, 1598, 1471, 1452, 1372, 1271, 1220, 732, 698 |
| 3p | Colorless oil | 95 | 259.1151 | 3291, 3029, 1647, 1613, 1601, 1506, 1453, 1265, 1247, 702 |
| 3q | Colorless oil | 90 | 275.1102 | 3392, 3028, 1501, 1451, 1266, 1240, 701 |
| 3r | Colorless oil | 88 | 259.1151 | 3287, 3020, 1644, 1601, 1485, 1269, 1240, 753 |
| 3s | Colorless oil | 86 | 279.0604 | 3298, 2968, 2928, 1644, 1596, 1488, 1455, 1268, 1233, 755 |
| 3t | Colorless oil | 84 | 263.0901 | 3305, 2969, 2928, 1643, 1613, 1589, 1486, 1267, 1234, 755 |
| 3u | Colorless oil | 82 | 263.0903 | 3305, 2969, 2928, 1603, 1506, 1487, 1269, 1226, 755 |
| 3v | Colorless oil | 90 | 279.0605 | 3294, 2969, 2928, 1642, 1599, 1482, 1416, 1268, 1234, 698 |
| 3w | Colorless oil | 85 | 251.1464 | 3271, 2927, 2852, 1609, 1581, 1487, 1451, 1271, 1231, 754 |
| 3x[ | Colorless oil | 88 | 169.0685 | 269, 2968, 2926, 1612, 1597, 1450, 1265, 1226, 699 |
| 4a[ | Colorless oil | 85 | 245.0997 |
Table 2 Appearance, yields, melting points, HRMS and IR data for compounds 3a—3x and 4a
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
|---|---|---|---|---|
| 3a | Colorless oil | 92 | 245.0996 | 3290, 3263, 3029, 1596, 1582, 1488, 1454, 1340, 1269, 1234, 754, 699 |
| 3b | Colorless oil | 90 | 259.1150 | 3289, 3062, 3029, 1596, 1582, 1487, 1454, 1339, 1270, 1236, 754, 699 |
| 3c | Colorless oil | 86 | 273.1312 | 3291, 3029, 1596, 1582, 1487, 1454, 1356, 1272, 1233, 754, 699 |
| 3d | Colorless oil | 84 | 287.1465 | 3292, 3029, 1596, 1582, 1487, 1454, 1341, 1271, 1234, 754, 699 |
| 3e | Colorless oil | 82 | 315.1779 | 3290, 3029, 1596, 1582, 1487, 1454, 1356, 1271, 1234, 753, 699 |
| 3f | Colorless oil | 87 | 273.1305 | 3291, 3029, 1597, 1583, 1488, 1454, 1366, 1271, 1234, 754, 699 |
| 3g | Colorless oil | 85 | 287.1467 | 3290, 3029, 1597, 1583, 1488, 1454, 1367, 1273, 1233, 754, 699 |
| 3h[ | Colorless oil | 88 | 259.1150 | 3290, 3029, 1598, 1583, 1489, 1454, 1366, 1271, 1235, 754, 699 |
| 3i | Colorless oil | 86 | 285.1308 | 3289, 3028, 1644, 1597, 1489, 1453, 1365, 1271, 1235, 754, 699 |
| 3j[ | Colorless oil | 91 | 307.1152 | 3289, 3028, 1644, 1599, 1493, 1454, 1341, 1272, 1234, 755, 699 |
| 3k | Colorless oil | 95 | 363.1777 | 3289, 3030, 1647, 1599, 1488, 1456, 1365, 1271, 1236, 756, 701 |
| 3l | Colorless oil | 86 | 341.0762 | 3296, 3029, 1644, 1597, 1490, 1454, 1365, 1272, 1233, 755, 699 |
| Compd. | Appearance | Yield*(%) | HRMS, m/z [M+H]+ | IR(film), |
| 3m | Colorless oil | 92 | 321.1305 | 3288, 3027, 1645, 1596, 1486, 1454, 1353, 1271, 1235, 754, 699 |
| 3n | Colorless oil | 90 | 301.1622 | 3246, 3028, 1655, 1600, 1586, 1494, 1483, 1363, 1268, 1231, 749, 701 |
| 3o | Colorless oil | 82 | 289.1257 | 3525, 3028, 1662, 1614, 1598, 1471, 1452, 1372, 1271, 1220, 732, 698 |
| 3p | Colorless oil | 95 | 259.1151 | 3291, 3029, 1647, 1613, 1601, 1506, 1453, 1265, 1247, 702 |
| 3q | Colorless oil | 90 | 275.1102 | 3392, 3028, 1501, 1451, 1266, 1240, 701 |
| 3r | Colorless oil | 88 | 259.1151 | 3287, 3020, 1644, 1601, 1485, 1269, 1240, 753 |
| 3s | Colorless oil | 86 | 279.0604 | 3298, 2968, 2928, 1644, 1596, 1488, 1455, 1268, 1233, 755 |
| 3t | Colorless oil | 84 | 263.0901 | 3305, 2969, 2928, 1643, 1613, 1589, 1486, 1267, 1234, 755 |
| 3u | Colorless oil | 82 | 263.0903 | 3305, 2969, 2928, 1603, 1506, 1487, 1269, 1226, 755 |
| 3v | Colorless oil | 90 | 279.0605 | 3294, 2969, 2928, 1642, 1599, 1482, 1416, 1268, 1234, 698 |
| 3w | Colorless oil | 85 | 251.1464 | 3271, 2927, 2852, 1609, 1581, 1487, 1451, 1271, 1231, 754 |
| 3x[ | Colorless oil | 88 | 169.0685 | 269, 2968, 2926, 1612, 1597, 1450, 1265, 1226, 699 |
| 4a[ | Colorless oil | 85 | 245.0997 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
|---|---|---|
| 3a | 7.40(d, J=7.4 Hz, 2H), 7.32—7.16(m, 5H), 7.05(dd, J=7.6, 1.2 Hz, 1H), 6.91(dd, J=8.0, 0.7 Hz, 1H), 6.88—6.82(m, 1H), 5.39(s, 1H), 2.49—2.43(m, 2H), 1.23(t, J=7.4 Hz, 3H) | 155.15, 139.31, 130.17, 129.21, 128.69, 128.67, 128.53, 128.51, 127.55, 125.16, 120.76, 117.60, 50.47, 26.36, 14.20 |
| 3b | 7.40(d, J=7.4 Hz, 2H), 7.34—7.29(m, 2H), 7.21(ddd, J=15.4, 10.6, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.95—6.89(m, 1H), 6.84(td, J=7.6, 1.0 Hz, 1H), 5.35(s, 1H), 2.48—2.39(m, 2H), 1.61(dd, J=14.6, 7.3 Hz, 2H), 0.95(t, J=7.4 Hz, 3H) | 155.22, 139.30, 130.18, 129.21, 128.66, 128.48, 127.53, 125.11, 120.70, 117.64, 50.97, 34.33, 22.32, 13.49 |
| 3c | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=10.0, 4.8 Hz, 3H), 7.26—7.11(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.90(m, 1H), 6.88—6.82(m, 1H), 5.35(s, 1H), 2.49—2.42(m, 2H), 1.63—1.51(m, 2H), 1.38—1.33(m, 2H), 0.85(t, J=7.3 Hz, 3H) | 155.23, 139.29, 130.19, 129.21, 128.67, 128.51, 128.49, 128.46, 127.54, 125.11, 120.71, 117.64, 51.04, 32.02, 21.01, 21.96, 13.64 |
| 3d | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=9.7, 5.1 Hz, 3H), 7.28—7.13(m, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.96—6.88(m, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.45(tq, J=10.7, 5.3 Hz, 2H), 1.57(dd, J=14.7, 7.3 Hz, 2H), 1.31—1.26(m, 4H), 0.85(t, J=7.1 Hz, 3H) | 155.23, 139.31, 130.19, 129.21, 128.67, 128.51, 128.48, 128.46, 127.53, 125.12, 120.71, 117.63, 51.05, 32.33, 30.99, 28.61, 22.23, 13.94 |
| 3e | 7.40(d, J=7.5 Hz, 2H), 7.31(dd, J=9.9, 4.9 Hz, 3H), 7.27—7.17(m, 2H), 7.03(dd, J=7.6, 1.2 Hz, 1H), 6.92(dd, J=8.0, 0.6 Hz, 1H), 6.86—6.82(m, 1H), 5.34(s, 1H), 2.45(tq, J=10.8, 5.3 Hz, 2H), 1.60—1.56(m, 2H), 1.32—1.23(m, 8H), 0.86(t, J=6.9 Hz, 3H) | 155.25, 139.29, 130.18, 129.21, 128.66, 128.54, 128.49, 127.53, 125.09, 120.70, 117.65, 51.08, 32.34, 31.67, 28.92, 28.80, 28.77, 22.61, 14.09 |
| 3f | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=13.8, 6.6 Hz, 3H), 7.21(ddd, J=11.3, 8.7, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.92(dd, J= 8.0, 0.6 Hz, 1H), 6.87—6.80(m, 1H), 5.31(s, 1H), 2.35(qd, J=12.5, 6.8 Hz, 2H), 1.83(dt, J=13.4, 6.7 Hz, 1H), 0.95(dd, J=8.0, 6.8 Hz, 6H) | 155.26, 139.36, 130.24, 129.22, 128.66, 128.51, 128.48, 128.45, 127.54, 125.12, 120.69, 117.65, 51.54, 41.27, 28.24, 22.22, 21.98 |
| 3g | 7.40(d, J=7.4 Hz, 2H), 7.36—7.26(m, 3H), 7.21(ddd, J=17.1, 11.2, 4.3 Hz, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.92(d, J=7.7 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.48—2.43(m, 2H), 1.61(dt, J=13.3, 6.6 Hz, 1H), 1.50—1.45(m, 2H), 0.84—0.81(m, 6H) | 155.22, 139.26, 130.19, 129.23, 128.66, 128.51, 128.49, 128.46, 127.54, 125.06, 120.71, 117.64, 51.03, 37.92, 30.36, 27.40, 22.32, 22.15 |
| 3h | 7.49—7.34(m, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.14(m, 2H), 7.04(dd, J=7.6, 1.2 Hz, 1H), 6.92(d, J=8.0 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.41(s, 1H), 2.77(dt, J=13.4, 6.7 Hz, 1H), 1.29—1.24(m, 6H) | 155.30, 139.28, 130.09, 129.19, 128.67, 128.48, 127.50, 125.11, 120.73, 117.73, 50.13, 35.76, 23.07, 22.85 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3i | 7.40(d, J=7.3 Hz, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.13(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.88(m, 1H), 6.85(dd, J=10.8, 4.1 Hz, 1H), 5.36(s, 1H), 2.91(p, J=7.0 Hz, 1H), 1.91(dt, J=12.3, 6.3 Hz, 2H), 1.72—1.70(m, 2H), 1.64—1.50(m, 4H) | 155.33, 139.37, 130.05, 129.15, 128.64, 128.46, 128.44, 127.47, 125.36, 120.70, 117.71, 51.40, 44.23, 33.65, 33.20, 24.88, 24.74 |
| 3j | 7.36(d, J=7.3 Hz, 2H), 7.30(t, J=7.3 Hz, 4H), 7.27—7.22(m, 2H), 7.18(dd, J=12.7, 4.5 Hz, 3H), 6.95(dd, J=7.7, 1.3 Hz, 1H), 6.90(d, J= 8.4 Hz, 2H), 6.86—6.79(m, 1H), 5.11(s, 1H), 3.59(d, J=3.2 Hz, 2H) | 155.03, 138.75, 137.28, 130.31 , 129.30, 129.01, 128.99, 128.76, 128.73, 127.65, 127.47, 124.86, 120.80, 117.60, 49.68, 36.54 |
| 3k | 7.34(dt, J=14.9, 7.8 Hz, 6H), 7.27—7.19(m, 2H), 7.15(d, J=8.2 Hz, 2H), 6.99(s, 1H), 6.96—6.90(m, 2H), 6.88—6.79(m, 1H), 5.14(s, 1H), 3.59(d, J=2.5 Hz, 2H), 1.32(s, 9H) | 155.14, 150.39, 138.73, 134.04, 130.35, 129.27, 128.73, 128.67, 128.64, 127.59, 125.59, 124.78, 120.70, 117.64, 49.97, 36.13, 34.57, 31.37 |
| 3l | 7.36—7.18(m, 8H), 7.11(d, J=8.4 Hz, 2H), 6.99(dd, J=7.6, 1.3 Hz, 1H), 6.93—6.81(m, 2H), 6.78(s, 1H), 5.12(s, 1H), 3.56(s, 2H) | 154.87, 138.61, 135.81, 133.18, 130.32, 130.29, 129.35,128.81, 128.75, 128.64, 127.70, 124.63, 120.88, 117.52, 49.65, 35.89 |
| 3m | 7.40—7.29(m, 4H), 7.26—7.17(m, 2H), 7.13—7.08(m, 4H), 6.96(s, 1H), 6.93—6.90(m, 2H), 6.83(t, J=7.4 Hz, 1H), 5.09(s, 1H), 3.56(d, J=5.1 Hz, 2H), 2.33(s, 3H) | 155.08, 138.74, 137.14, 134.11, 130.31, 129.41, 129.26 , 128.90, 128.79, 128.71, 127.61, 124.87, 120.74, 117.62, 49.64, 36.21, 21.20 |
| 3n | 7.61(s, 1H), 7.42(d, J=7.4 Hz, 2H), 7.34(t, J=7.4 Hz, 2H), 7.30—7.21(m, 2H), 6.74(dd, J=7.2, 5.3 Hz, 2H), 5.35(s, 1H), 2.46(q, J=7.4 Hz, 2H), 1.44(s, 9H), 1.25(t, J=7.3 Hz, 3H) | 154.38, 138.88, 138.29, 128.82, 128.63, 128.04, 127.56, 126.66, 125.19, 119.80, 77.36, 77.05, 76.73, 51.54, 34.93, 29.76, 26.27, 14.24 |
| 3o | 7.47(d, J=7.4 Hz, 2H), 7.28(t, J=7.5 Hz, 2H), 7.22—7.08(m, 2H), 6.81(t, J=8.0 Hz, 1H), 6.71(dd, J=8.0, 1.1 Hz, 1H), 5.98(s, 1H), 5.66(s, 1H), 4.10—4.02(m, 2H), 2.44(q, J=7.4 Hz, 2H), 1.41(t, J=7.0 Hz, 3H), 1.23(t, J=7.4 Hz, 3H) | 145.71, 143.18, 141.48, 128.38, 128.35, 127.33, 126.88, 121.02, 119.63, 110.09, 64.57, 46.17, 26.31, 14.92, 14.31 |
| 3p | 7.40(d, J=7.5 Hz, 2H), 7.31(t, J=7.4 Hz, 2H), 7.26—7.22(m, 1H), 7.12(s, 1H), 7.00(dd, J=8.2, 1.7 Hz, 1H), 6.83(dd, J=8.1, 4.9 Hz, 2H), 5.34(s, 1H), 2.48(dd, J=7.4, 4.0 Hz, 2H), 2.21(s, 3H), 1.25(t, J=7.4 Hz, 3H) | 152.92, 139.33, 130.56, 129.85, 129.72, 128.65, 128.45, 127.48, 124.56, 117.49, 50.76, 26.36, 20.57, 14.16 |
| 3q | 7.41(d, J=7.4 Hz, 2H), 7.32(dd, J=10.1, 4.7 Hz, 2H), 7.25(t, J=7.2 Hz, 1H), 6.86(d, J=8.8 Hz, 1H), 6.76(dd, J=8.8, 3.1 Hz, 2H), 6.63(d, J=3.0 Hz, 1H), 5.34(s, 1H), 3.70(s, 3H), 2.48(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 153.54, 148.85, 139.05, 128.66, 128.64, 128.49, 128.44, 127.56, 126.25, 118.26, 115.74, 113.95, 55.66, 50.59, 26.35, 14.18 |
| 3r | 7.54—7.51(m, 1H), 7.29(s, 1H), 7.23—7.11(m, 4H), 6.94(ddd, J=11.9, 7.9, 1.0 Hz, 2H), 6.83—6.80(m, 1H), 5.52(s, 1H), 2.51(q, J=7.4 Hz, 2H), 2.39(s, 3H), 1.27(t, J=7.4 Hz, 3H) | 155.18, 137.18, 136.28, 130.73, 129.94, 128.98, 128.62, 127.48, 126.47, 125.15, 120.79, 117.44, 46.90, 26.47, 19.31, 14.16 |
| 3s | 7.34(d, J=8.5 Hz, 2H), 7.22(ddd, J=11.3, 8.7 Hz, 4.7 Hz, 2H), 7.08—7.03(m, 2H), 6.93—6.85(m, 2H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 154.95, 137.91, 133.29, 129.97, 129.84, 129.35, 128.78, 124.75, 120.86, 117.63, 49.68, 26.37, 14.12 |
| 3t | 7.30—7.26(m, 1H), 7.21(ddd, J=12.9, 9.8, 4.7 Hz, 2H), 7.14(dd, J=10.1, 2.0 Hz, 1H), 7.06(dd, J=7.6, 1.3 Hz, 1H), 7.01(s, 1H), 6.97—6.86(m, 3H), 5.38(s, 1H), 2.49(qd, J=7.4, 1.6 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 164.17, 161.72, 154.88, 142.07(d, J=6.9 Hz), 130.07(d, J=8.3 Hz), 129.99, 129.37, 124.76, 124.12(d, J=2.8 Hz), 120.89, 117.58, 115.63, 115.41, 114.56, 114.35, 49.70, 26.36, 14.12 |
| 3u | 7.39—7.35(m, 2H), 7.22(td, J=8.1, 1.6 Hz, 1H), 7.17(s, 1H), 7.05—6.98(m, 3H), 6.93(dd, J =8.0, 0.8 Hz, 1H), 6.87(td, J=7.5, 1.0 Hz, 1H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.7 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 162.04(d, J=245 Hz), 155.05, 134.99(d, J=3 Hz), 130.12, 130.03(d, J=2.6 Hz), 129.32, 124.87, 120.80, 117.69, 115.59, 115.38, 49.78, 26.35, 14.10 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3v | 7.40—7.25(m, 6H), 7.16(dd, J=8.6, 2.6 Hz, 1H), 7.03(d, J=2.5 Hz, 1H), 6.86(d, J=8.6 Hz, 1H), 5.31(s, 1H), 2.49(dd, J=13.7, 7.0 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 153.82, 138.45, 129.70, 129.03, 128.80, 128.76, 128.41, 128.36, 128.32, 127.82, 126.81, 125.44, 119.00, 50.32, 29.71, 26.41, 14.10 |
| 3w | 7.64(s, 1H), 7.18—7.14(m, 1H), 6.96(dd, J=7.5, 1.3 Hz, 1H), 6.86(d, J=7.7, 1H), 6.80(dd, J=11.5, 4.1 Hz, 1H), 3.78(d, J=9.1 Hz, 1H), 2.29(qd, J=7.4, 1.5 Hz, 2H), 2.14(d, J=12.8 Hz, 1H), 1.87—1.85(m, 1H), 1.74(dd, J=10.4, 2.7 Hz, 1H), 1.63—1.60(m, 2H), 1.41(d, J=13.0 Hz, 1H), 1.23(ddd, J=15.7, 8.6, 5.2 Hz, 1H), 1.15—1.03(m, 6H), 0.92—0.90(m, 1H) | 155.51, 131.14, 128.68, 124.72, 119.88, 117.59, 55.16, 41.45, 32.07, 32.02, 26.28, 26.16, 14.24 |
| 3x | 7.20(t, J=7.5 Hz, 1H), 7.08(d, J=7.2 Hz, 1H), 6.97—6.79(m, 2H), 6.75(s, 1H), 3.82(s, 2H), 2.42(q, J=7.3 Hz, 2H), 1.26—1.21(m, 3H) | 155.51, 130.45, 129.11, 122.44, 120.52, 117.20, 32.38 , 24.76, 14.23 |
| 4a | 7.40(d, J=7.4 Hz, 2H), 7.25(td, J=17.2, 7.3 Hz, 5H), 6.74(d, J=8.5 Hz, 2H), 5.79(s, 1H), 5.12(s, 1H), 2.37(q, J=7.4 Hz, 2H), 1.19(t, J=7.4 Hz, 3H) | 154.72, 141.81, 133.61, 129.56, 128.56, 128.26, 127.09, 115.49, 53.17, 26.27, 14.25 |
Table 3 1H NMR and 13C NMR data for compounds 3a—3x and 4a
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
|---|---|---|
| 3a | 7.40(d, J=7.4 Hz, 2H), 7.32—7.16(m, 5H), 7.05(dd, J=7.6, 1.2 Hz, 1H), 6.91(dd, J=8.0, 0.7 Hz, 1H), 6.88—6.82(m, 1H), 5.39(s, 1H), 2.49—2.43(m, 2H), 1.23(t, J=7.4 Hz, 3H) | 155.15, 139.31, 130.17, 129.21, 128.69, 128.67, 128.53, 128.51, 127.55, 125.16, 120.76, 117.60, 50.47, 26.36, 14.20 |
| 3b | 7.40(d, J=7.4 Hz, 2H), 7.34—7.29(m, 2H), 7.21(ddd, J=15.4, 10.6, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.95—6.89(m, 1H), 6.84(td, J=7.6, 1.0 Hz, 1H), 5.35(s, 1H), 2.48—2.39(m, 2H), 1.61(dd, J=14.6, 7.3 Hz, 2H), 0.95(t, J=7.4 Hz, 3H) | 155.22, 139.30, 130.18, 129.21, 128.66, 128.48, 127.53, 125.11, 120.70, 117.64, 50.97, 34.33, 22.32, 13.49 |
| 3c | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=10.0, 4.8 Hz, 3H), 7.26—7.11(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.90(m, 1H), 6.88—6.82(m, 1H), 5.35(s, 1H), 2.49—2.42(m, 2H), 1.63—1.51(m, 2H), 1.38—1.33(m, 2H), 0.85(t, J=7.3 Hz, 3H) | 155.23, 139.29, 130.19, 129.21, 128.67, 128.51, 128.49, 128.46, 127.54, 125.11, 120.71, 117.64, 51.04, 32.02, 21.01, 21.96, 13.64 |
| 3d | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=9.7, 5.1 Hz, 3H), 7.28—7.13(m, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.96—6.88(m, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.45(tq, J=10.7, 5.3 Hz, 2H), 1.57(dd, J=14.7, 7.3 Hz, 2H), 1.31—1.26(m, 4H), 0.85(t, J=7.1 Hz, 3H) | 155.23, 139.31, 130.19, 129.21, 128.67, 128.51, 128.48, 128.46, 127.53, 125.12, 120.71, 117.63, 51.05, 32.33, 30.99, 28.61, 22.23, 13.94 |
| 3e | 7.40(d, J=7.5 Hz, 2H), 7.31(dd, J=9.9, 4.9 Hz, 3H), 7.27—7.17(m, 2H), 7.03(dd, J=7.6, 1.2 Hz, 1H), 6.92(dd, J=8.0, 0.6 Hz, 1H), 6.86—6.82(m, 1H), 5.34(s, 1H), 2.45(tq, J=10.8, 5.3 Hz, 2H), 1.60—1.56(m, 2H), 1.32—1.23(m, 8H), 0.86(t, J=6.9 Hz, 3H) | 155.25, 139.29, 130.18, 129.21, 128.66, 128.54, 128.49, 127.53, 125.09, 120.70, 117.65, 51.08, 32.34, 31.67, 28.92, 28.80, 28.77, 22.61, 14.09 |
| 3f | 7.40(d, J=7.4 Hz, 2H), 7.31(dd, J=13.8, 6.6 Hz, 3H), 7.21(ddd, J=11.3, 8.7, 4.4 Hz, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.92(dd, J= 8.0, 0.6 Hz, 1H), 6.87—6.80(m, 1H), 5.31(s, 1H), 2.35(qd, J=12.5, 6.8 Hz, 2H), 1.83(dt, J=13.4, 6.7 Hz, 1H), 0.95(dd, J=8.0, 6.8 Hz, 6H) | 155.26, 139.36, 130.24, 129.22, 128.66, 128.51, 128.48, 128.45, 127.54, 125.12, 120.69, 117.65, 51.54, 41.27, 28.24, 22.22, 21.98 |
| 3g | 7.40(d, J=7.4 Hz, 2H), 7.36—7.26(m, 3H), 7.21(ddd, J=17.1, 11.2, 4.3 Hz, 2H), 7.03(dd, J=7.6, 1.1 Hz, 1H), 6.92(d, J=7.7 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.35(s, 1H), 2.48—2.43(m, 2H), 1.61(dt, J=13.3, 6.6 Hz, 1H), 1.50—1.45(m, 2H), 0.84—0.81(m, 6H) | 155.22, 139.26, 130.19, 129.23, 128.66, 128.51, 128.49, 128.46, 127.54, 125.06, 120.71, 117.64, 51.03, 37.92, 30.36, 27.40, 22.32, 22.15 |
| 3h | 7.49—7.34(m, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.14(m, 2H), 7.04(dd, J=7.6, 1.2 Hz, 1H), 6.92(d, J=8.0 Hz, 1H), 6.84(t, J=7.5 Hz, 1H), 5.41(s, 1H), 2.77(dt, J=13.4, 6.7 Hz, 1H), 1.29—1.24(m, 6H) | 155.30, 139.28, 130.09, 129.19, 128.67, 128.48, 127.50, 125.11, 120.73, 117.73, 50.13, 35.76, 23.07, 22.85 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3i | 7.40(d, J=7.3 Hz, 3H), 7.30(t, J=7.4 Hz, 2H), 7.26—7.13(m, 2H), 7.03(dd, J=7.6, 1.3 Hz, 1H), 6.97—6.88(m, 1H), 6.85(dd, J=10.8, 4.1 Hz, 1H), 5.36(s, 1H), 2.91(p, J=7.0 Hz, 1H), 1.91(dt, J=12.3, 6.3 Hz, 2H), 1.72—1.70(m, 2H), 1.64—1.50(m, 4H) | 155.33, 139.37, 130.05, 129.15, 128.64, 128.46, 128.44, 127.47, 125.36, 120.70, 117.71, 51.40, 44.23, 33.65, 33.20, 24.88, 24.74 |
| 3j | 7.36(d, J=7.3 Hz, 2H), 7.30(t, J=7.3 Hz, 4H), 7.27—7.22(m, 2H), 7.18(dd, J=12.7, 4.5 Hz, 3H), 6.95(dd, J=7.7, 1.3 Hz, 1H), 6.90(d, J= 8.4 Hz, 2H), 6.86—6.79(m, 1H), 5.11(s, 1H), 3.59(d, J=3.2 Hz, 2H) | 155.03, 138.75, 137.28, 130.31 , 129.30, 129.01, 128.99, 128.76, 128.73, 127.65, 127.47, 124.86, 120.80, 117.60, 49.68, 36.54 |
| 3k | 7.34(dt, J=14.9, 7.8 Hz, 6H), 7.27—7.19(m, 2H), 7.15(d, J=8.2 Hz, 2H), 6.99(s, 1H), 6.96—6.90(m, 2H), 6.88—6.79(m, 1H), 5.14(s, 1H), 3.59(d, J=2.5 Hz, 2H), 1.32(s, 9H) | 155.14, 150.39, 138.73, 134.04, 130.35, 129.27, 128.73, 128.67, 128.64, 127.59, 125.59, 124.78, 120.70, 117.64, 49.97, 36.13, 34.57, 31.37 |
| 3l | 7.36—7.18(m, 8H), 7.11(d, J=8.4 Hz, 2H), 6.99(dd, J=7.6, 1.3 Hz, 1H), 6.93—6.81(m, 2H), 6.78(s, 1H), 5.12(s, 1H), 3.56(s, 2H) | 154.87, 138.61, 135.81, 133.18, 130.32, 130.29, 129.35,128.81, 128.75, 128.64, 127.70, 124.63, 120.88, 117.52, 49.65, 35.89 |
| 3m | 7.40—7.29(m, 4H), 7.26—7.17(m, 2H), 7.13—7.08(m, 4H), 6.96(s, 1H), 6.93—6.90(m, 2H), 6.83(t, J=7.4 Hz, 1H), 5.09(s, 1H), 3.56(d, J=5.1 Hz, 2H), 2.33(s, 3H) | 155.08, 138.74, 137.14, 134.11, 130.31, 129.41, 129.26 , 128.90, 128.79, 128.71, 127.61, 124.87, 120.74, 117.62, 49.64, 36.21, 21.20 |
| 3n | 7.61(s, 1H), 7.42(d, J=7.4 Hz, 2H), 7.34(t, J=7.4 Hz, 2H), 7.30—7.21(m, 2H), 6.74(dd, J=7.2, 5.3 Hz, 2H), 5.35(s, 1H), 2.46(q, J=7.4 Hz, 2H), 1.44(s, 9H), 1.25(t, J=7.3 Hz, 3H) | 154.38, 138.88, 138.29, 128.82, 128.63, 128.04, 127.56, 126.66, 125.19, 119.80, 77.36, 77.05, 76.73, 51.54, 34.93, 29.76, 26.27, 14.24 |
| 3o | 7.47(d, J=7.4 Hz, 2H), 7.28(t, J=7.5 Hz, 2H), 7.22—7.08(m, 2H), 6.81(t, J=8.0 Hz, 1H), 6.71(dd, J=8.0, 1.1 Hz, 1H), 5.98(s, 1H), 5.66(s, 1H), 4.10—4.02(m, 2H), 2.44(q, J=7.4 Hz, 2H), 1.41(t, J=7.0 Hz, 3H), 1.23(t, J=7.4 Hz, 3H) | 145.71, 143.18, 141.48, 128.38, 128.35, 127.33, 126.88, 121.02, 119.63, 110.09, 64.57, 46.17, 26.31, 14.92, 14.31 |
| 3p | 7.40(d, J=7.5 Hz, 2H), 7.31(t, J=7.4 Hz, 2H), 7.26—7.22(m, 1H), 7.12(s, 1H), 7.00(dd, J=8.2, 1.7 Hz, 1H), 6.83(dd, J=8.1, 4.9 Hz, 2H), 5.34(s, 1H), 2.48(dd, J=7.4, 4.0 Hz, 2H), 2.21(s, 3H), 1.25(t, J=7.4 Hz, 3H) | 152.92, 139.33, 130.56, 129.85, 129.72, 128.65, 128.45, 127.48, 124.56, 117.49, 50.76, 26.36, 20.57, 14.16 |
| 3q | 7.41(d, J=7.4 Hz, 2H), 7.32(dd, J=10.1, 4.7 Hz, 2H), 7.25(t, J=7.2 Hz, 1H), 6.86(d, J=8.8 Hz, 1H), 6.76(dd, J=8.8, 3.1 Hz, 2H), 6.63(d, J=3.0 Hz, 1H), 5.34(s, 1H), 3.70(s, 3H), 2.48(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 153.54, 148.85, 139.05, 128.66, 128.64, 128.49, 128.44, 127.56, 126.25, 118.26, 115.74, 113.95, 55.66, 50.59, 26.35, 14.18 |
| 3r | 7.54—7.51(m, 1H), 7.29(s, 1H), 7.23—7.11(m, 4H), 6.94(ddd, J=11.9, 7.9, 1.0 Hz, 2H), 6.83—6.80(m, 1H), 5.52(s, 1H), 2.51(q, J=7.4 Hz, 2H), 2.39(s, 3H), 1.27(t, J=7.4 Hz, 3H) | 155.18, 137.18, 136.28, 130.73, 129.94, 128.98, 128.62, 127.48, 126.47, 125.15, 120.79, 117.44, 46.90, 26.47, 19.31, 14.16 |
| 3s | 7.34(d, J=8.5 Hz, 2H), 7.22(ddd, J=11.3, 8.7 Hz, 4.7 Hz, 2H), 7.08—7.03(m, 2H), 6.93—6.85(m, 2H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.0 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 154.95, 137.91, 133.29, 129.97, 129.84, 129.35, 128.78, 124.75, 120.86, 117.63, 49.68, 26.37, 14.12 |
| 3t | 7.30—7.26(m, 1H), 7.21(ddd, J=12.9, 9.8, 4.7 Hz, 2H), 7.14(dd, J=10.1, 2.0 Hz, 1H), 7.06(dd, J=7.6, 1.3 Hz, 1H), 7.01(s, 1H), 6.97—6.86(m, 3H), 5.38(s, 1H), 2.49(qd, J=7.4, 1.6 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 164.17, 161.72, 154.88, 142.07(d, J=6.9 Hz), 130.07(d, J=8.3 Hz), 129.99, 129.37, 124.76, 124.12(d, J=2.8 Hz), 120.89, 117.58, 115.63, 115.41, 114.56, 114.35, 49.70, 26.36, 14.12 |
| 3u | 7.39—7.35(m, 2H), 7.22(td, J=8.1, 1.6 Hz, 1H), 7.17(s, 1H), 7.05—6.98(m, 3H), 6.93(dd, J =8.0, 0.8 Hz, 1H), 6.87(td, J=7.5, 1.0 Hz, 1H), 5.36(s, 1H), 2.47(qd, J=7.4, 2.7 Hz, 2H), 1.25(t, J=7.4 Hz, 3H) | 162.04(d, J=245 Hz), 155.05, 134.99(d, J=3 Hz), 130.12, 130.03(d, J=2.6 Hz), 129.32, 124.87, 120.80, 117.69, 115.59, 115.38, 49.78, 26.35, 14.10 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(CDCl3, 100 MHz), δ |
| 3v | 7.40—7.25(m, 6H), 7.16(dd, J=8.6, 2.6 Hz, 1H), 7.03(d, J=2.5 Hz, 1H), 6.86(d, J=8.6 Hz, 1H), 5.31(s, 1H), 2.49(dd, J=13.7, 7.0 Hz, 2H), 1.26(t, J=7.4 Hz, 3H) | 153.82, 138.45, 129.70, 129.03, 128.80, 128.76, 128.41, 128.36, 128.32, 127.82, 126.81, 125.44, 119.00, 50.32, 29.71, 26.41, 14.10 |
| 3w | 7.64(s, 1H), 7.18—7.14(m, 1H), 6.96(dd, J=7.5, 1.3 Hz, 1H), 6.86(d, J=7.7, 1H), 6.80(dd, J=11.5, 4.1 Hz, 1H), 3.78(d, J=9.1 Hz, 1H), 2.29(qd, J=7.4, 1.5 Hz, 2H), 2.14(d, J=12.8 Hz, 1H), 1.87—1.85(m, 1H), 1.74(dd, J=10.4, 2.7 Hz, 1H), 1.63—1.60(m, 2H), 1.41(d, J=13.0 Hz, 1H), 1.23(ddd, J=15.7, 8.6, 5.2 Hz, 1H), 1.15—1.03(m, 6H), 0.92—0.90(m, 1H) | 155.51, 131.14, 128.68, 124.72, 119.88, 117.59, 55.16, 41.45, 32.07, 32.02, 26.28, 26.16, 14.24 |
| 3x | 7.20(t, J=7.5 Hz, 1H), 7.08(d, J=7.2 Hz, 1H), 6.97—6.79(m, 2H), 6.75(s, 1H), 3.82(s, 2H), 2.42(q, J=7.3 Hz, 2H), 1.26—1.21(m, 3H) | 155.51, 130.45, 129.11, 122.44, 120.52, 117.20, 32.38 , 24.76, 14.23 |
| 4a | 7.40(d, J=7.4 Hz, 2H), 7.25(td, J=17.2, 7.3 Hz, 5H), 6.74(d, J=8.5 Hz, 2H), 5.79(s, 1H), 5.12(s, 1H), 2.37(q, J=7.4 Hz, 2H), 1.19(t, J=7.4 Hz, 3H) | 154.72, 141.81, 133.61, 129.56, 128.56, 128.26, 127.09, 115.49, 53.17, 26.27, 14.25 |
| [1] | Kondo T., Mitsudo T. A., Chem.Rev., 2000, 100(8), 3205—3220 |
| [2] | Feng M., Tang B., Liang S. H., Jiang X., Curr. Top. Med.Chem.,2016, 16(17), 1200—1216 |
| [3] | Ilardi E. A., Vitaku E., Najardarson J. T., J. Med.Chem., 2014, 57(7), 2832—2842 |
| [4] | Smith B. R., Eastman C. M., Nijardarson., J. Med.Chem., 2014, 57(23), 9764—9773 |
| [5] | Wei Y. Z., Tan X. Z., Chem. Res. Chinese Universities,2017, 33(5), 731—735 |
| [6] | Zhong S. M., Zheng M., Pu S. X., Xing Y. B., Li K. Z., Wang H., Chem. Res. Chinese Universities,2017, 33(6), 979—985 |
| [7] | Cinar M. E., Ozturk T., Chem.Rev., 2015, 115(9), 3036—3140 |
| [8] | Takimiya K., Osaka I., Mori T., Nakano M., Acc. Chem.Res., 2014, 47(5), 1493—1502 |
| [9] | Scott K. A., Njardarson J. T., Top. Curr.Chem., 2018, 376, 5—38 |
| [10] | Hammond M. L., Zambias R. A., Chang M. N., Jensen N. P., McDonald J., Thompson K., Boulton D. A., Kopka I. K., Hand K. M., Opas E. E., Luell S., Bach T., Davies P., MacIntyre D. E., Bonney R. J., Humes J. L., J. Med.Chem.,1990, 33(3), 908—918 |
| [11] | Katritzky A. R., Zhang Z., Lan X., Lang H., J. Org.Chem., 1994, 59(7), 1900—1903 |
| [12] | Modica E., Zanaletti R., Freccero M., Mella M., J. Org.Chem.,2001, 66(1), 41—52 |
| [13] | Shaikh A. K., Cobb A. J. A., Varvounis G., Org.Lett., 2012, 14(2), 584—587 |
| [14] | Lau C. K., Williams H. W. R., Tardiff S., Dufresne C., Scheigetz J., Belanger P., Can. J.Chem., 1989, 67, 1384—1387 |
| [15] | Guo W., Wu B., Zhou X., Chen P., Wang X., Zhou Y. G., Liu Y., Li C., Angew. Chem. Int.Ed., 2015, 54(15), 4522—4526 |
| [16] | Guo W., Wu B., Zhou X., Chen P., Wang X., Zhou Y. G., Liu Y., Li C.,Angew. Chem. Int.Ed., 2015, 54(15), 4605—4609 |
| [17] | Lai Z., Sun J., Synlett.,2016, 27(4), 555—558 |
| [18] | Pathak T. P., Sigman M. S., J. Org.Chem.,2011, 76(22), 9210—9215 |
| [19] | Will N. J., Bray C. D., Chem. Eur.J.,2012, 18(30), 9160—9173 |
| [20] | Caruana L., Fochi M., Bernardi L., Molecules,2015, 20(7), 11733—11764 |
| [21] | Wang Z., Sun J., Synthesis,2015, 47(23), 3629—3644 |
| [22] | Water R. W. V. D., Pettus T. R. R., Tetrahedron,2002, 58(27), 5367—5405 |
| [23] | Kulikov A., Arumugam S., Popik V. V., J. Org.Chem.,2008, 73(19), 7611—7615 |
| [24] | Mattson A. E., Scheidt K. A., J. Am. Chem.Soc.,2007, 129(15), 4508—4509 |
| [25] | Luan Y., Schaus S. E., J. Am. Chem.Soc., 2012, 134(49), 19965—19968 |
| [26] | Chen M. W., Gao L. L., Ye Z. S., Jiang G. F., Zhou Y. G., Chem.Commun.,2013, 49, 1660—1662 |
| [27] | Bai W. J., David J. G., Feng Z. G., Weaver M. G., Wu K. L., Pettus T. R. R., Acc. Chem.Res., 2014, 47(12), 3655—3664 |
| [28] | Zhao W., Wang Z., Chu B., Sun J., Angew. Chem. Int.Ed., 2015, 54(6), 1910—1913 |
| [29] | Huang Y., Hayashi T., J. Am. Chem.Soc.,2015, 137(24), 7556—7559 |
| [30] | Wan Z., Ai F., Wang Z., Zhao W., Zhu G., Lin Z., Sun J., J. Am. Chem.Soc., 2015, 137(1), 383—389 |
| [31] | Wu B., Yu Z., Gao X., Lan Y., Zhou Y. G., Angew. Chem. Int.Ed.,2017, 56(14), 4006—4010 |
| [32] | Chen P., Wang K., Guo W., Liu X., Liu Y., Li C., Angew. Chem. Int.Ed.,2017, 56(13), 3689—3693 |
| [33] | Nising C. F., Brase S., Chem. Soc.Rev., 2008, 37, 1218—1228 |
| [34] | Nising C. F., Brase S., Chem. Soc.Rev., 2012, 41, 988—999 |
| [35] | Heravi M. M., Hajiabbasi P., Mol.Diversity,2014, 18(2), 411—439 |
| [36] | Thirupathi N., Tung C. H., Xu Z. H.,Adv. Synth.Catal., 2018, 360(18), 3585—3589 |
| [37] | Corma A., Gonzalez-Arellano C., Iglesias M., Sanchez F., App. Catal. A:Gen.,2010, 375(1), 49—54 |
| [38] | Lee W. Z., Tseng H. S., Wang T. L., Tsai H. L., Kuo T. S.,Organometallics,2010, 29(13), 2874—2881 |
| [39] | Badoiu A., Bernardinelli G., Besnard C., Kundig E. P., Org. Biomol.Chem., 2010, 8, 193—200 |
| [40] | O’Byrne A., Murray C., Keegan D., Palacio C., Evans P., Morgan B. S., Org. Biomol.Chem., 2010, 8, 539—545 |
| [41] | Firouzabadi H., Iranpoor N., Abbasi M., Adv. Synth.Catal., 2009, 351, 755—766 |
| [42] | Rajabi F., Razavi S., Luque R., Green Chem.,2010, 12, 786—789 |
| [43] | Inoue T., Inoue S., Sato K., Bull. Chem. Soc.Jpn.,1990, 63(4), 1062—1068 |
| [44] | Lanzi M., Merad J., Boyarskaya D V., Maestri G., Allain C., Masson Géraldine., Org.Lett., 2018, 20(17), 5247—5250 |
| [45] | Basha R. S., Chen C. W., Reddy D. M., Lee C. F., Chem-Asian J.,2018,13(17), 2475—2483 |
| [1] | WANG Zhengwen, GAO Fengxiang, CAO Han, LIU Shunjie, WANG Xianhong, WANG Fosong. Synthesis and Property of CO2 Copolymer⁃based UV-curable Polymer [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220236. |
| [2] | ZHANG Wanbin, WANG Yanmeng, WANG Shaowu, TONG Xin, HAN Xiaoqian, ZHANG Ce, ZHANG Guanghua, ZHU Xiuzhong. Synthesis of Poly(allyl glycidyl ether) Bearing Alkyl Functional Side Groups and Its Plasticizing and Antistatic Effects for PVC [J]. Chem. J. Chinese Universities, 2021, 42(9): 2961. |
| [3] | LI Aiju, WANG Yuxi, LU Shaoyong, LIU Kun. Ligand Exchange of Gold Nanoparticles with Thiol-terminated Polystyrene† [J]. Chem. J. Chinese Universities, 2018, 39(3): 552. |
| [4] | YAN Dong, TONG Mengliang. Metal-free Thiolation of C(sp3)—S Bond Adjacent to an Oxygen Atom† [J]. Chem. J. Chinese Universities, 2016, 37(2): 269. |
| [5] | HU Jing-Han, YAN Nong-Ping, CHEN Juan-Juan, LI Jian-Bin. Synthesis of Azosalicylic Aldehyde of Benzoyl Hydrazone Based Sensor and It’s Colorimetric Sensing Properties for Cyanide Anions in Aqueous Solutions [J]. Chem. J. Chinese Universities, 2013, 34(6): 1368. |
| [6] | YU Hai-Feng, LIAO Pei-Qiu. Odorless and Efficient Thioacetalization Reaction of Oximes [J]. Chem. J. Chinese Universities, 2012, 33(09): 1969. |
| [7] | ZHANG Yong, SHI Wen-Fang. Synthesis and Photopolymerization Properties of Self-initiated Photopolymerized Acrylate Oligomers [J]. Chem. J. Chinese Universities, 2012, 33(03): 635. |
| [8] | JI Yong, HU Liang, ZHANG Rui, LI Yi-Zhi, ZUO Jing-Lin*, YOU Xiao-Zeng. Syntheses, Structures and Electrochemical Properties of Dinuclear Au(Ⅰ) Complexes Including Crown Ether Annelated Dithiolate Ligands [J]. Chem. J. Chinese Universities, 2011, 32(3): 545. |
| [9] | ZENG Zhi, GUAN Shao-Wei, ZHU Shi-Yang, DAI Zhong-Ming, JIANG Zhen-Hua*. Synthesis of New Aromatic Trithiol for the Thiol–ene Ultraviolet-Curable Formulations [J]. Chem. J. Chinese Universities, 2011, 32(2): 407. |
| [10] | XIE Bin*, ZHANG Xiu-Lan, ZOU Li-Ke, WANG Jun, LAI Chuan, WU Yu, .... Synthesis, Characterization and Crystal Structure of Complex {[Cu(hmtade)][Ni(dmit)2]}2·4DMSO [J]. Chem. J. Chinese Universities, 2009, 30(12): 2337. |
| [11] | ZHU Lun-Yu1,2, XU Kai2, WANG Hong1,2, LIU Peng1,2, CHEN De-Hong1,2, AI Hao1,2, CHEN Ming-Cai1*. Synthesis and Characterization of Rare Earth(Lanthanum) Thiolate [J]. Chem. J. Chinese Universities, 2008, 29(9): 1786. |
| [12] | HUANG Bin, CAO Ze-Xing*. A Theoretical Study on Complexes of Transition Metals Complex with Extended-TTF Dithiolate Ligands [J]. Chem. J. Chinese Universities, 2008, 29(8): 1625. |
| [13] | ZHANG Xiao-Peng1,2, LU Shi-Wei2*. Synthesis of Thiocarbamates via Selenium-mediated Carbonylation of Aniline with Thiols [J]. Chem. J. Chinese Universities, 2008, 29(6): 1137. |
| [14] | WANG Xin, ZHENG Chao , ZHANG Xin-Ge , WANG Zhen , LI Chao-Xing*. Synthesis and Characterization of Novel Bioadhesive Material Thiolated Chitosan [J]. Chem. J. Chinese Universities, 2008, 29(1): 206. |
| [15] | XU Ning, WANG Rui, DU Fu-Sheng, LI Zi-Chen*. Synthesis of Thiol-Terminated Poly(ε-caprolactone) [J]. Chem. J. Chinese Universities, 2007, 28(9): 1791. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||