

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (3): 497.doi: 10.7503/cjcu20170668
• Physical Chemistry • Previous Articles Next Articles
CHEN Jie1,2, ZHAO Yingxian1, ZHANG Yongming3, ZHANG Shengjian1,*( ), YAN Shan1, ZHAO Hong1, LI Xianming3, YING Liyan1
), YAN Shan1, ZHAO Hong1, LI Xianming3, YING Liyan1
Received:2017-10-11
Online:2018-03-10
Published:2018-01-13
Contact:
ZHANG Shengjian
E-mail:zsj@nit.zju.edu.cn
Supported by:CLC Number:
TrendMD:
CHEN Jie, ZHAO Yingxian, ZHANG Yongming, ZHANG Shengjian, YAN Shan, ZHAO Hong, LI Xianming, YING Liyan. Effects of TMPDO and 4-NOH-TMPD on H2O2/HTS Catalytic System for Free Radicals Generation and Its Application†[J]. Chem. J. Chinese Universities, 2018, 39(3): 497.
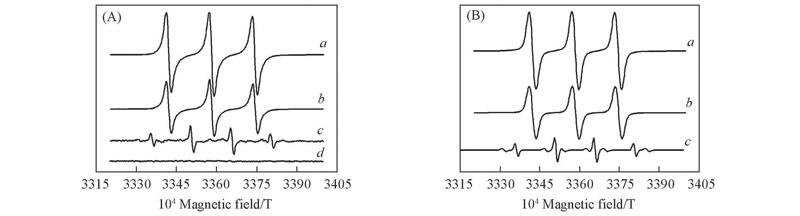
Fig.1 Solution EPR spectra of experiment(A) and simulation(B)(A) a. HTS/H2O2/4-NOH-TMPD; b. HTS/H2O2/TMPDO; c. HTS/H2O2; d. 0.08 mol/L DMPO. (B) Reaction condition: a. 0.1 g HTS+10.0 g H2O2+0.1 g 4-NOH-TMPD; b. 0.1 g HTS+10.0 g H2O2+0.1 g TMPDO; c. 0.1 g HTS+10.0 g H2O2; 70 ℃, 1 h.
| System | Intensity of HO·(Gain) | Intensity of ·(Gain) |
|---|---|---|
| HTS/H2O2 | 1.5×106 | 2.5×105 |
| HTS/H2O2/TMPDO | 5.0×106 | |
| HTS/H2O2/4-NOH-TMPD | 7.0×106 |
Table 1 Intensities of HO· and · in different systems
| System | Intensity of HO·(Gain) | Intensity of ·(Gain) |
|---|---|---|
| HTS/H2O2 | 1.5×106 | 2.5×105 |
| HTS/H2O2/TMPDO | 5.0×106 | |
| HTS/H2O2/4-NOH-TMPD | 7.0×106 |
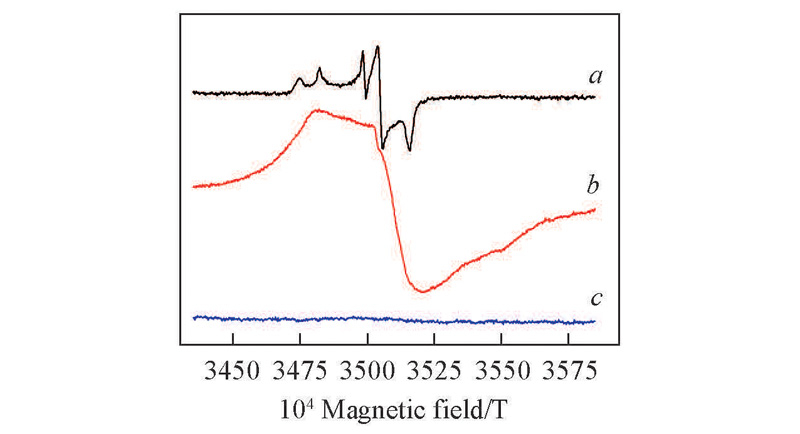
Fig.2 EPR spectra of HTS in different systemsa. HTS/H2O2; b. HTS/H2O2/4-NOH-TMPD; c. HTS/H2O2/4-NOH-TMPD/acetone oxime. Reaction condition: a. 0.1 g HTS+10.0 g H2O2; b. 0.1 g HTS+10.0 g H2O2+0.1 g NOH-TMPD; c. 0.1 g HTS+10.0 g H2O2+0.1 g NOH-TMPD, 2 g acetone oxime. 70 ℃, 1 h.
| Systems | gx | gy | gz1(A) | gz2(B) | gz3 | g | Ref. |
|---|---|---|---|---|---|---|---|
| HTS/H2O2 | 2.00157 | 2.00805 | 2.02525 | 2.02089 | 2.01153 | This work | |
| TS/H2O2 | 2.0024 | 2.0085 | 2.0259 | 2.0230 | [26] | ||
| TS/H2O2 | 2.0033 | 2.0095 | 2.0269 | 2.0234 | [24] | ||
| TS/H2O2(Water solvent) | 2.0023 | 2.0090 | 2.0266 | 2.0236 | [15] | ||
| TS/H2O2(CH3OH solvent) | 2.0023 | 2.0090 | 2.0260 | 2.0235 | 2.0220 | [15] | |
| HTS/4-NOH-TMPD/H2O2 | 1.98284 | 2.00524 | 2.02246 | 2.00963 | This work |
Table 2 EPR parameters of Ti—OO-· in different systems
| Systems | gx | gy | gz1(A) | gz2(B) | gz3 | g | Ref. |
|---|---|---|---|---|---|---|---|
| HTS/H2O2 | 2.00157 | 2.00805 | 2.02525 | 2.02089 | 2.01153 | This work | |
| TS/H2O2 | 2.0024 | 2.0085 | 2.0259 | 2.0230 | [26] | ||
| TS/H2O2 | 2.0033 | 2.0095 | 2.0269 | 2.0234 | [24] | ||
| TS/H2O2(Water solvent) | 2.0023 | 2.0090 | 2.0266 | 2.0236 | [15] | ||
| TS/H2O2(CH3OH solvent) | 2.0023 | 2.0090 | 2.0260 | 2.0235 | 2.0220 | [15] | |
| HTS/4-NOH-TMPD/H2O2 | 1.98284 | 2.00524 | 2.02246 | 2.00963 | This work |
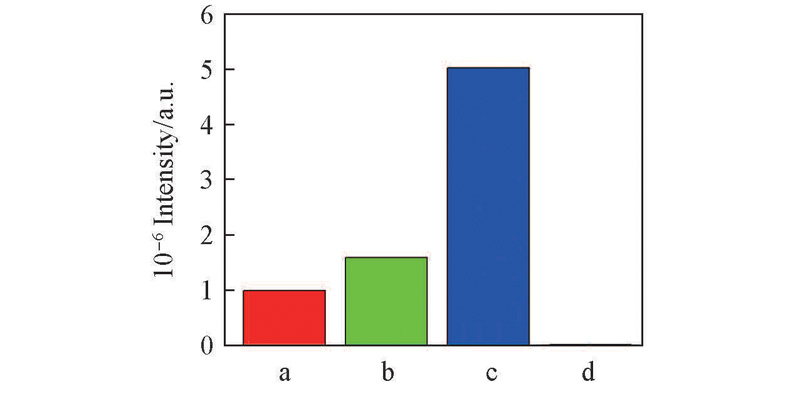
Fig.5 Relative EPR intensity of TiOO-· species (A and B) in HTS at different systemsa. HTS/H2O2(A); b. HTS/H2O2(B);c. HTS/4-NOH-TMPD/H2O2(A);d. HTS/4-NOH-TMPD/H2O2(B).
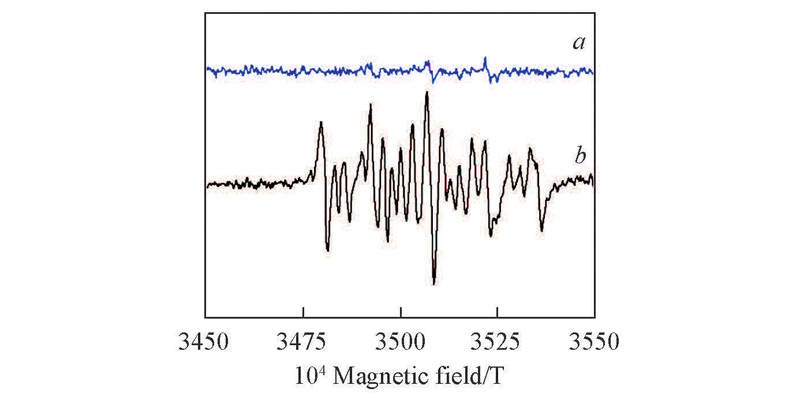
Fig.6 Solution EPR spectra of different systems after acetone ammoximationa. Fresh HTS, m(HTS):m(acetone oxime)=0.6, n(H2O2):n(acetone oxime)=4.0; b. fresh HTS, m(HTS):m(4-NOH-TMPD)=2.0, m(HTS):m(acetone oxime)=0.6, n(H2O2):n(acetone)=4.0; DMPO: 0.08 mol/L. Reaction conditions: n(NH3):n(H2O2)=1: 2, m(H2O2):m(acetone oxime)=1.0, 70 ℃, NH3 and H2O2 were dropped into reactor within 1 h, and reaction time was 15 min.
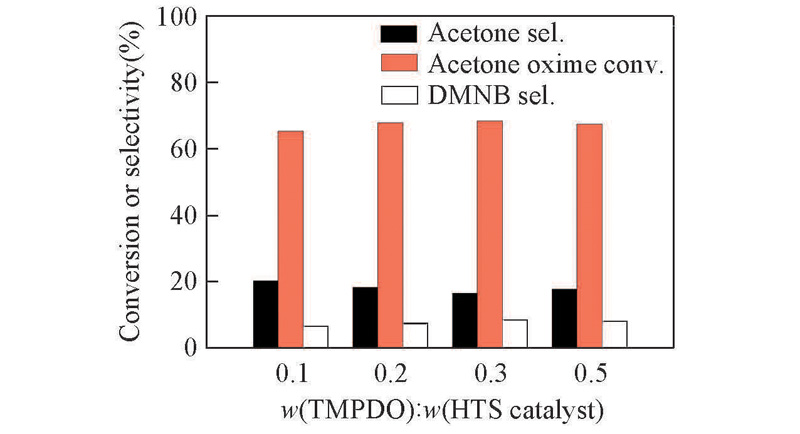
Fig.8 Effect of TMPDO on the oxidation coupling reaction of acetone oxime catalyzed by HTSReaction conditions: 0.1 mol acetone oxime, 50.0 g H2O, n(H2O2):n(acetone oxime)=4.0, m(HTS):m(acetone oxime)=0.3, n(H2O2) :n(NH3) =4.0, dripping H2O2 and NH3 into the solution at fixed pH 10—11 and 90 ℃, keeping isothermal reaction for 2 h.
| [1] | Taramasso M., Perego G., Notari B., Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides US 4410501, 1983-10-18 |
| [2] | Bhaumik A., Mukherjee P., Kumar R., J. Catal., 1998, 178(1), 101—107 |
| [3] | Le Bars J., Dakka J., Sheldon R. A., Appl. Catal. A: Gen., 1996, 136(1), 69—80 |
| [4] | Tatsumi T., Jappar N., J. Catal., 1996, 161(2), 570—576 |
| [5] | Chen R. Z., Bu Z., Li Z. H., Zhong Z. X., Jin W. Q., Xing W. H., Chem. Eng. J., 2010, 156(2), 418—422 |
| [6] | Bordiga S., Bonino F., Damin A., Lamberti C., Phys. Chem. Chem. Phys., 2007, 9(35), 4854—4878 |
| [7] | Spanó E., Tabacchi G., Gamba A., Fois E., J. Phys. Chem. B, 2006, 110(43), 21651—21661 |
| [8] | Panyaburapa W., Nanok T., Limtrakul J., J. Phys. Chem. C, 2007, 111(8), 3433—3441 |
| [9] | Wang L. L., Xiong G., Su J., Li P., Guo H. C., J. Phys. Chem. C, 2012, 116(16), 9122—9131 |
| [10] | Yoon C. W., Hirsekorn K. F., Neidig M. L., Yang X. Z., Tilley T. D., ACS Catal., 2011, 1(12), 1665—1678 |
| [11] | Cordeiro P. J., Tilley T. D., ACS Catal., 2011, 1(5), 455—467 |
| [12] | Geobaldo F., Bordiga S., Zecchina A., Giamello E., Leofanti G., Petrini G., Catal. Lett., 1992, 16(1/2), 109—115 |
| [13] | Rode C. V., Nehete U. N., Dongare M. K., Catal. Commun., 2003, 4(8), 365—369 |
| [14] | Tresp H., Hammer M. U., Winter J., Weltmann K. D., Reuter S., J. Phys. Appl. Phys., 2013, 46(43), 1—9 |
| [15] | Srinivas D., Manikandan P., Laha S. C., Kumar R., Ratnasamy P., J. Catal., 2003, 217(1), 160—171 |
| [16] | Chaudhari K., Srinivas D., Ratnasamy P., J. Catal., 2001, 203(1), 25—32 |
| [17] | Zhao Q., Bao X. H., Wang Y., Lin L. W., Li G., Guo X. W., Wang X. S., J. Mol. Catal. A: Chem., 2000, 157(1/2), 265—268 |
| [18] | Yan S., Zhang S. J., Zhao Y. X., Li X. M., Zhang Y. M., Zhang H., Wang J., Fu J. Q., Chem. J. Chinese Universities, 2016, 37(5), 946—955 |
| (严山, 张胜建, 赵迎宪, 李显明, 张永明, 张洪, 王健, 符建琼.高等学校化学学报,2016, 37(5), 946—955) | |
| [19] | Zhang S. J., Zhao Y. X., Zhang H., Chin. J. Org. Chem., 2012, 32(4), 786—789 |
| (张胜建, 赵迎宪, 张洪.有机化学,2012, 32(4), 786—789) | |
| [20] | Rockenbauer A., Clément J. L., Culcasi M., Mercier A., Tordo P., Pietri S., J. Phy. Chem. A, 2007, 111(23), 4950—4957 |
| [21] | Clément J. L., Ferré N., Siri D., KarouiH., Rockenbauer A., Tordo P., J. Org. Chem., 2005, 70(4), 1198—1203 |
| [22] | Wang Y., Zhang S. J., Zhao Y. X., Lin M., J. Mol. Catal. A: Chem., 2014, 385, 1—6 |
| [23] | Su J., Yang L. S., Liu R. N., Lin H. F., Chinese J. Catal., 2014, 35(5), 622—630 |
| [24] | Bonoldi L., Busetto C., Congiu A., Marra G., Ranghino G., Salvalaggio M., Spano G., Giamello E., Spectrochim. Acta Part A, 2002, 58(6), 1143—1154 |
| [25] | Clerici M. G., Top. Catal., 2001, 15(2—4), 257—263 |
| [26] | Heinrich S., Plettig M., Klemm E., Catal. Lett., 2011, 141(2), 251—258 |
| [27] | Antcliff K. L., Murphy D. M., Griffiths E., Giamello E., Phys. Chem. Chem. Phys., 2003, 5(9), 4306—4316 |
| [28] | Xu Y. Z., Liu J. G., Petrochem. Techno., 2002, 31(4), 316—321 |
| (徐元植, 刘嘉庚.`石油化工,2002, 31(4), 316—321) | |
| [29] | Lin M., Zhu B., Shu X. T., Wang X. Q., Petrochem. Techno., 2005, 34(Suppl.), 377—379 |
| (林民, 朱斌, 舒兴田, 汪燮卿.石油化工,2005, 34(增刊), 377—379) | |
| [30] | Li D. M., Shi F., Deng Y. Q., Tetrahedron Lett., 2004, 45(36), 6791—6794 |
| [1] | LIU Huiqiang, PENG Chao, CHEN Ning, LIU Yangping. Novel Fluorescent/EPR Difunctional Probe for Detecting Hypochlorite† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1542. |
| [2] | AN Pengjiao, YU Nannan, SUN Ruisheng, SUI Xiaofang, SONG Yuguang. Characterization of the Interaction Between Esterified TAM Radical and Bovine Serum Albumin [J]. Chem. J. Chinese Universities, 2017, 38(8): 1354. |
| [3] | ZHOU Jun1,3, WU Ke2, SUN Yue3, CONG Jian-Bo2, WANG Chang-Zhen2, CHANG Xiang3, XIAN Hong2, ZHU Yong-Fa1*. Development of a Quantitative Method for Analyzing Gas-phase Free Radicals in Mainstream Cigarette Smoke [J]. Chem. J. Chinese Universities, 2007, 28(10): 1846. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||