

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (12): 2220.doi: 10.7503/cjcu20170390
• Physical Chemistry • Previous Articles Next Articles
Received:2017-06-19
Online:2017-12-10
Published:2017-11-21
Contact:
WU Xiaojing
E-mail:wuxiaojing@ustc.edu
TrendMD:
WU Xiaojing, LIU Azuan. Studies of Raman Spectra and Theoretical Calculation of MnCl2/DMSO Solution[J]. Chem. J. Chinese Universities, 2017, 38(12): 2220.
| Species | d(S=O)/nm | d(C—S)/nm | ΔG/(kJ·mol-1) |
|---|---|---|---|
| DMSO | 0.1511 | 0.1837 | |
| (DMSO)2 | 0.1522 | 0.1831 | |
| [Mn(DMSO)]2+ | 0.1667 | 0.1813 | -765.8 |
| [Mn(DMSO)2]2+ | 0.1606 | 0.1811 | -469.9 |
| [Mn(DMSO)3]2+ | 0.1593 | 0.1812 | -239.1 |
| [Mn(DMSO)4]2+ | 0.1584 | 0.1813 | -187.7 |
| [Mn(DMSO)5]2+ | 0.1570 | 0.1817 | -42.6 |
| [Mn(DMSO)6]2+ | 0.1561 | 0.1818 | -51.5 |
Table 1 Bond parameters and the changes of Gibbs free energy(ΔG) of different configurations
| Species | d(S=O)/nm | d(C—S)/nm | ΔG/(kJ·mol-1) |
|---|---|---|---|
| DMSO | 0.1511 | 0.1837 | |
| (DMSO)2 | 0.1522 | 0.1831 | |
| [Mn(DMSO)]2+ | 0.1667 | 0.1813 | -765.8 |
| [Mn(DMSO)2]2+ | 0.1606 | 0.1811 | -469.9 |
| [Mn(DMSO)3]2+ | 0.1593 | 0.1812 | -239.1 |
| [Mn(DMSO)4]2+ | 0.1584 | 0.1813 | -187.7 |
| [Mn(DMSO)5]2+ | 0.1570 | 0.1817 | -42.6 |
| [Mn(DMSO)6]2+ | 0.1561 | 0.1818 | -51.5 |
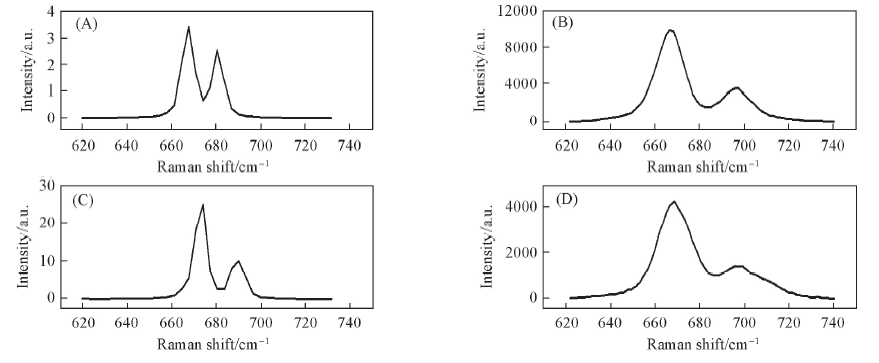
Fig.10 Theory and experiment spectra of C—S stretching vibration(A) The cumulative peak of DMSO and (DMSO)2; (B)experimental spectra of pure DMSO; (C) the cumulative peak of [Mn(DMSO)n]2+(n=1—6); (D) experimental spectra of 1.0 mol/L MnCl2/DMSO.
| [1] | Saytzeff A., Ann. Chem. Pharm., 1967, 144(148), 15—17 |
| [2] | Clark T., Murray J. S., Lane P., Politzer P., J. Mol. Model., 2008, 14(8), 689—697 |
| [3] | Kirillov S. A., Gorobets M. I., Gafurov M. M., Ataev M. B., J. Phys. Chem. B, 2013, 117(32), 9439—9448 |
| [4] | Shikata T., Sugimoto N., J. Phys. Chem. A, 2012, 116(3), 990—999 |
| [5] | Gores H.J., Barthel J., Zugmann S., Handbook of Battery Materials, Second Edition, Wiley-VCH, 2011, 526—626 |
| [6] | Gafurov M. M., Ataev M. B., Rabadanov K. S., Gorobets M. I., Russ. J. Phys. Chem. A,2015, 89(4), 639—643 |
| [7] | Gafurov M. M., Kirillov S. A., Gorobets M. I., Rabadanov K. S., J. Appl. Spectro., 2015, 81(6), 824—830 |
| [8] | Gorobets M. I., Ataev M. B., Gafurov M. M., Kirillov S. A., J. Molecular Liquids, 2015, 205, 98—109 |
| [9] | Wu X. J., Pan Y., Song L. L., Chem. J. Chinese Universities, 2010, 31(9), 1741—1746 |
| (吴晓静, 潘燕, 宋路路. 高等学校化学学报, 2010, 31(9), 1741—1746) | |
| [10] | Wu X. J., Dai Y., Zhang N., Li J., Acta Phys. Chim. Sinica, 2011, 27(11), 2535—2540 |
| (吴晓静, 代云, 张楠, 李静. 物理化学学报, 2011, 27(11), 2535—2540) | |
| [11] | Wu X. J., Yu X. H., Liu A. Z., Jiang W. G., Spectrosc. Spect. Anal., 2017, 37(2), 513—516 |
| (吴晓静, 于学会, 刘阿钻, 蒋卫国. 光谱学与光谱分析, 2017, 37(2), 513—516) | |
| [12] | Wu X. J., Xu X. N., J. Chin. Chem. Soc., 2009, 67(6), 535—540 |
| (吴晓静, 许晓娜. 化学学报, 2009, 67(6), 535—540) | |
| [13] | Kloss A. A., Fawcett W. R., J. Chem. Soc., 1998, 94(11), 1587—1591 |
| [14] | Xuan X., Wang J., Zhao Y., Zhu J., J. Raman Spectrosc., 2007, 38, 865—872 |
| [15] | Xue X. P., Zhang H. C., Wang J. J., Wang H. Q., J. Chin. Chem. Soc., 2004, 62(10), 940—945 |
| (轩小朋, 张虎成, 王键吉, 汪汉卿. 化学学报, 2004, 62(10), 940—945) | |
| [16] | Kirillov S. A., Gorobets M. I., Gafurov M. M., Ataev M. B., Russ. J. Phys. Chem. A,2014, 88(1), 175—177 |
| [17] | Fawcett W. R., Kloss A. A., J. Chem. Soc., Faraday Trans., 1996, 92, 3333—3337 |
| [18] | Zavitsas A. A., J. Phys. Chem. B, 2005, 109(43), 20636—20640 |
| [19] | Zhang Y., Wang Y., Zhang S., Li G. Q., Chem. J. Chinese Universities, 2016, 37(12), 2260—2267 |
| (张宇, 王翀, 张帅, 李根全. 高等学校化学学报, 2016, 37(12), 2260—2267) | |
| [20] | Sun L., Wang H. Q., Wu M., Li H. F., Chem. J. Chinese Universities, 2016, 37(10), 1840—1848 |
| (孙林, 王怀谦, 吴梦, 李慧芳. 高等学校化学学报, 2016, 37(10), 1840—1848) |
| [1] | SU Yingli, REN Haisheng, LI Xiangyuan. Application of New Nonequilibrium Solvation Theory in Electronic Spectra of Organic Dyes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2254. |
| [2] | ZHAO Yuhui, LI Mingle, LONG Saran, FAN Jiangli, PENG Xiaojun. Spectroscopic Characterization of Solvation Effect for a Polarity-Sensitive BDP [J]. Chem. J. Chinese Universities, 2020, 41(9): 2018. |
| [3] | TONG Ti*, ZHAO Yangyang, FU Yilin, SHAN Guiye. Special Surface Enhanced Raman Scattering on Lung Cancer Tissues Based on Au/Cu Nanorods Substrate† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1536. |
| [4] | WANG Kai, WANG Qinglei, YAN Tingting, LIN Aolei. Raman Spectroscopic Study on High Pressure-induced Phase Transition of D,L-Mandelic† [J]. Chem. J. Chinese Universities, 2015, 36(2): 381. |
| [5] | MING Meijun, BI Tingjun, LI Xiangyuan. Nonequilibrium Solvation Theory Based on Constrained Equilibrium Principle and Its Applications† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2256. |
| [6] | OUYANG Bing, XUE Jiadan, ZHENG Xuming. Excited State Dynamics of γ-Crotonolactone: Resonance Raman Spectroscopy and Complete Active Space Self-consistent Field(CASSCF) Study† [J]. Chem. J. Chinese Universities, 2015, 36(10): 1995. |
| [7] | LIN Xiaomin, ZHU Lili, HAN Jian, LIU Xiaomei. Microstructure and Electrical Properties of Solid Electrolytes Ce0.9Er0.1-xPrx [J]. Chem. J. Chinese Universities, 2015, 36(1): 61. |
| [8] | YANG Youwen, ZHU Wenbin, LI Tianying, MA Dongming, CHEN Dong. Controlled Fabrication and Spectral Characterization of InSb Nanowire Arrays† [J]. Chem. J. Chinese Universities, 2014, 35(3): 466. |
| [9] | HOU Haiyun, HUANG Yinrong, BAI Bofeng, YANG Jing. Volumetric Properties and Molecular Interactions of Binary Mixtures Imidazolium Acetates-ethanol at 293.15 K† [J]. Chem. J. Chinese Universities, 2014, 35(1): 121. |
| [10] | LIU Chun-Yu, WANG Shao-Yan, XU Shu-Ping, XU Wei-Qing. SERS and EEM Fluorescence Spectral Distinction of Sudan Ⅰ and Paprika Red in Food [J]. Chem. J. Chinese Universities, 2013, 34(11): 2505. |
| [11] | ZHOU Jing, LI Liang, HUANG Feng-Xian, SHEN Hong-Zhi, YANG Hang, ZHOU Qiang, WANG Wen-Quan, XU Da-Peng. In-situ Raman Spectra and X-ray Diffraction Study on Pressure-induced Phase Transition in Columbite ZnNb2O6 [J]. Chem. J. Chinese Universities, 2013, 34(10): 2383. |
| [12] | XU Zong-Ping, ZHAO Yan-Ying, WANG Hui-Gang, ZHENG Xu-Ming. Resonance Raman Spectroscopic and Density Functional Theory Investigations of Excited State Structural Dynamics of 2-Acetyl-1-methylpyrrole(2-Ac-NMP) and Its Solvent Effect [J]. Chem. J. Chinese Universities, 2012, 33(04): 772. |
| [13] | SUN Yuan-Yuan, SHAN Ning, WANG Bin-Bin, LIAN Wen-Hui, YU Miao, SHI Tong-Shun. Synthesis and Characterization of Bis-porphyrins with Different Substituents [J]. Chem. J. Chinese Universities, 2012, 33(03): 496. |
| [14] | LIN Xiao-Min*, SHUN Jia-Ling, YAN Shi, ZHU Li-Li, SU Wen-Hui. Synthesis and Characterization of Solid Solution Ce0.8Gd0.2–xPrxO1.9 [J]. Chem. J. Chinese Universities, 2011, 32(2): 231. |
| [15] | WU Xiao-Jing*, PAN Yan, SONG Lu-Lu. Ionic Solvation of NaNO3 and NaClO4 in N,N-Dimethylformamide Solvent [J]. Chem. J. Chinese Universities, 2010, 31(9): 1741. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
