

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (1): 101.doi: 10.7503/cjcu20160457
• Physical Chemistry • Previous Articles Next Articles
ZOU Tao, YI Qingfeng*( ), ZHANG Yuanyuan, DENG Zhongliang, LEI Ming, ZHOU Xiulin
), ZHANG Yuanyuan, DENG Zhongliang, LEI Ming, ZHOU Xiulin
Received:2016-06-27
Online:2017-01-10
Published:2016-12-15
Contact:
YI Qingfeng
E-mail:yqfyy2001@hnust.edu.cn
Supported by:CLC Number:
TrendMD:
ZOU Tao, YI Qingfeng, ZHANG Yuanyuan, DENG Zhongliang, LEI Ming, ZHOU Xiulin. A New Formic Acid/Iron Ion Fuel Cell†[J]. Chem. J. Chinese Universities, 2017, 38(1): 101.
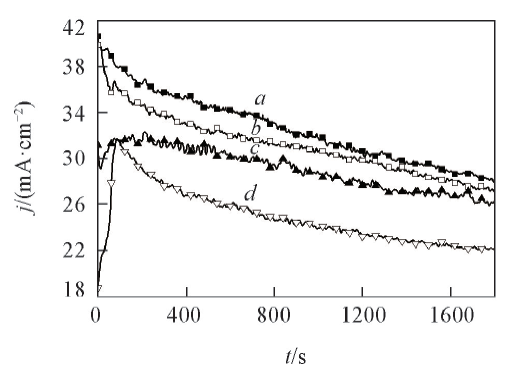
Fig.3 Chronoamperometric responses of the prepared catalysts in 1.0 mol/L NaOH+0.5 mol/L HCOOH solution at -0.2 Va. PdSn/β-CD-MWCNT; b. PdSn/MWCNT;c. Pd/β-CD-MWCNT; d. Pd/C.
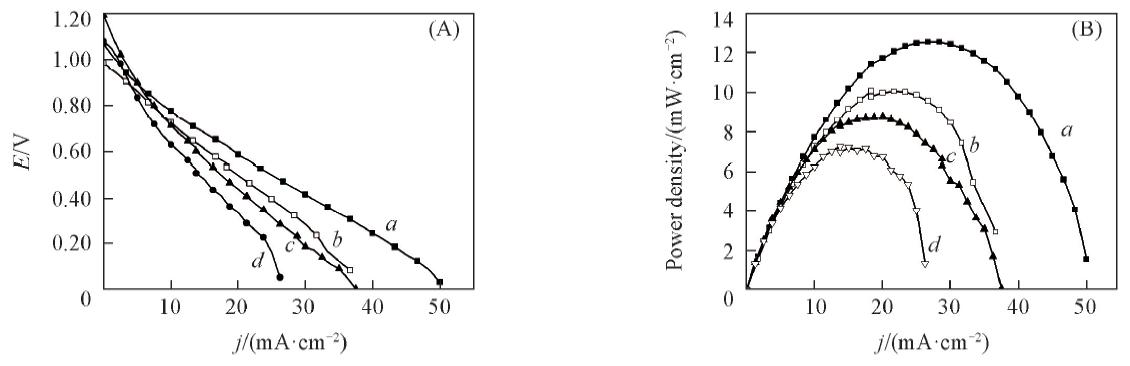
Fig.5 Formic acid/ Fe3+ cell polarization curves(A) and plots of power density vs. cell current density(B) with different anode catalystsAnolyte: 1.0 mol/L NaOH+0.5 mol/L HCOOH; catholyte 0.5 mol/L NaCl+0.5 mol/L FeCl3, cathode: carbon powder. Anode catalyst: a. PdSn/β-CD-MWCNT; b. PdSn/MWCNT; c. Pd/β-CD-MWCNT; d. Pd/C.
| [1] | Rice C., Ha S., Masel R. I., Waszezuk P., Wieekowski A., Baxnard T., J. Power Source,2002, 111, 83—89 |
| [2] | Tang Y. W., Zhang L. L., Wang X., Bao J. C., Zhou Y. M., Lu L. D., Lu T. H., Chem. Res. Chinese Universities,2009, 25(2), 239—242 |
| [3] | Geng X. W., Wu B., Gao Y., Chem. J. Chinese Universities,2012, 33(9), 2025—2029 |
| (耿小为, 邬冰, 高颖. 高等学校化学学报, 2012, 33(9), 2025—2029) | |
| [4] | Li H. Z., Shen J. Z., Yang G. X., Tang Y. W., Lu T. H., Chem. J. Chinese Universities,2011, 32(7), 1445—1450 |
| (李焕芝, 沈娟章, 杨改秀, 唐亚文, 陆天虹. 高等学校化学学报, 2011, 32(7), 1445—1450) | |
| [5] | Zhang S. X., Qing M., Zhang H., Tian Y. N., Eletrochem. Commun., 2009, 11, 2249—2252 |
| [6] | Yi Q. F., Huang W., Liu X. P., Xu G. R., Zhou Z. H., Chen A. C., J. Electroanal. Chem., 2008, 619/620(15), 197—205 |
| [7] | Zhang L. L., Tang Y. W., Bao J. C., Li C., J. Power Sources,2006, 162, 177—179 |
| [8] | Niu F. J., Yi Q. F., Liu Y. Q., Chin. J. Nonferrous Metals,2011, 21(8), 1974—1979 |
| (牛凤娟, 易清风, 刘云清. 中国有色金属学报, 2011, 21(8), 1974—1979) | |
| [9] | Kim J., Momma T., Osaka T., J. Power Sources,2009, 189, 999—1002 |
| [10] | Yi Q. F., Sun L. Z., Liu X. P., Nie H. D., Fuel,2013, 111, 88—95 |
| [11] | Duan D. H., You X., Liang J. W., Liu S. B., Wang Y. F., Electrochim. Acta,2015, 176, 1126—1135 |
| [12] | Ge X., Li B. J., Chen T., Wang G. Y., Mater. Rev., 2010, 20(4), 11—15 |
| (葛鑫, 李碧静, 陈彤, 王公应. 材料导报, 2010, 20(4), 11—15) | |
| [13] | Shen H. M., Ji H. B., Wu H. K., Shi H. X., Chin. J. Org. Chem., 2014, 34, 1549—1572 |
| (沈海民, 纪红兵, 武宏科, 史宏鑫. 有机化学, 2014, 34, 1549—1572) | |
| [14] | Jia Y. J., Jiang J. C., Sun K., Lu T. H., J. Fuel Chem. Tech., 2011, 10, 792—795 |
| (贾羽洁, 蒋剑春, 孙康, 陆天虹. 燃料化学学报, 2011, 10, 792—795) | |
| [15] | Yuan R., Preparation and Study of Pt-Bi Cathode Catylysts of Direct Methanol Full Cell, Beijing University of Technology, Beijing, 2005 |
| (袁嵘. 直接甲醇燃料电池阴极催化剂Pt-Bi制备及电催化性能研究, 北京: 北京工业大学, 2005) | |
| [16] | Xu J. B., Zhao T. S., Li Y. S., Yang W. W., Hydrogen Energy,2010, 35, 9693—9700 |
| [17] | Shobha T., Aravinda C. L., Bera P., Mayanna S. M., Mater. Chem. Phys., 2003, 80, 656—661 |
| [18] | Modibedi R. M., Masombuka T., Mathe M. K., Hydrogen Energy,2011, 36, 4664—4672 |
| [19] | Yi Q. F., Chen Q. H., Electrochim. Acta,2015, 182, 96—103 |
| [20] | Shen P. K., Xu C. W., Electrochem. Commun., 2006, 8, 184—188 |
| [21] | Ma D. N., Chen W., Jiao L. S., Tang Y. W., Liu C. P., Xing W., Lu T. H., Chin. J. Inorg. Chem., 2008, 11, 1803—1806 |
| (马德娜, 陈卫, 焦连升, 唐亚文, 刘长鹏, 邢巍, 陆天虹. 无机化学学报, 2008, 11, 1803—1806) | |
| [22] | Yang W., Study on Nitrogen-Doped Carbon Supported Non Precious Metal Method Catalysts for Direct Methanaol Fuel Cell, South China University of Technology, Guangzhou, 2012 |
| (杨伟. 直接甲醇燃料电池阴极用氮碳载非贵金属催化剂研究, 广州: 华南理工大学, 2012) | |
| [23] | Zhang Q., Study of Fe3+/Fe2+ Electrolyte and Electrode Materials in Redox Flow Battery, Central South University, Changsha, 2014 |
| (张清. 应用于液流电池的铁电解液及电极材料研究, 长沙: 中南大学, 2014) | |
| [24] | Huang J., Studies on Fe(Ⅱ)/Fe(Ⅲ) Redox Couple in Cathodic Eletrolyte for a Novel Redox Battery Application, Central South University, Changsha, 2014 |
| (黄佳. 应用于新型氧化还原电池正极液的Fe(Ⅲ)/Fe(Ⅱ)电解质的研究, 长沙: 中南大学, 2014) | |
| [25] | Herrera E., Wiithrich R., Mandin P., Fóti G., Comninellis C., J. Appl. Electrochem., 2009, 39, 1379—1384 |
| [26] | Xu P. C., Xu H. F., Dong Y. M., Shen Y., Wu X. X., Chin. J. Power Sources,2015, 7, 1438—1441 |
| (许鹏程, 徐洪峰, 董一鸣, 沈阳, 吴晓欣. 电源技术, 2015, 7, 1438—1441) | |
| [27] | Yi Q. F., Zou T., Zhang Y. Y., Liu X. P., Xu G. R., Nie H. D., Zhou X. L., J. Power Sources,2016, 321, 219—225 |
| [28] | Liu Z., Zhang X., Electrochem. Commun., 2009, 11, 1667—1670 |
| [29] | Morales-Acosta D., Ledesma-Garcia J., A. Godinez Luis, Rodríguez H. G., Álvarez-Contreras L., Arriaga L.G., J. Power Sources,2010, 195, 461—465 |
| [30] | Zhu Y., Khan Z., Masel R. I., J. Power Sources,2005, 139, 15—20 |
| [1] | QIU Xinsheng, WU Qin, SHI Daxin, ZHANG Yaoyuan, CHEN Kangcheng, LI Hansheng. Preparation and High Temperature Fuel Cell Performance of Ionic Crosslinked Sulfonated Polyimides for Proton Exchange Membranes [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220140. |
| [2] | CHEN Changli, MI Wanliang, LI Yujing. Research Progress of Single Atom Catalysts in Electrochemical Hydrogen Cycling [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220065. |
| [3] | LUO Bian, ZHOU Fen, PAN Mu. Study on Preparation and Accessibility of Hierarchical Porous Carbon Supported Platinum Catalyst [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210853. |
| [4] | LIU Jie, LI Jinsheng, BAI Jingsen, JIN Zhao, GE Junjie, LIU Changpeng, XING Wei. Constructing a Water-blocking Interlayer Containing Sulfonated Carbon Tubes to Reduce Concentration Polarization in Direct Methanol Fuel Cells [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220420. |
| [5] | PU Yangyang, NING Cong, LU Yao, LIU Lili, LI Na, HU Zhaoxia, CHEN Shouwen. Preparation and Characterizations of Cross-linked Sulfonated Poly(ether ether ketone)/Partially Fluorinated Sulfonated Poly(aryl ether sulfone) Blend Membranes [J]. Chem. J. Chinese Universities, 2021, 42(6): 2002. |
| [6] | CAO Kaiyue, PENG JinWu, LI Hongbin, SHI Chengying, WANG Peng, LIU Baijun. High-temperature Proton Exchange Membranes Based on Cross-linked Polybenzimidazole/hyperbranched-polymer Blends [J]. Chem. J. Chinese Universities, 2021, 42(6): 2049. |
| [7] | WANG Yuemin, MENG Qinglei, WANG Xian, GE Junjie, LIU Changpeng, XING Wei. Enhancement of Performance of Fe-N-C Catalysts by Copper and Sulfur Doping for the Oxygen Reduction Reaction [J]. Chem. J. Chinese Universities, 2020, 41(8): 1843. |
| [8] | LIANG Minhui, WANG Peng, LI Hongbin, LI Tianyang, CAO Kaiyue, PENG Jinwu, LIU Zhenchao, LIU Baijun. Preparation of High-temperature Proton Exchange Membranes Based on Semi-interpenetrating Polymer Networks [J]. Chem. J. Chinese Universities, 2020, 41(12): 2845. |
| [9] | HUA Tao, LI Shengnan, LI Fengxiang, WANG Haonan. Treatment of Naphthalene by Microbial Electrochemical System and the Analysis of Microbial Communities † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1964. |
| [10] | YU Yancun, WANG Xian, GE Junjie, LIU Changpeng, XING Wei. Promoted Formic Acid Electrooxidation Using PdNx/C Catalyst Prepared with Hyperbranched Polymer† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1433. |
| [11] | ZHU Yuxin,HARAGIRIMANA Alphonse,LU Yao,BUREGEYA Ingabire Providence,NING Cong,LI Na,HU Zhaoxia,CHEN Shouwen. Preparation and Properties of Filling-type Sulfonated Poly(arylene ether sulfone)/Poly(ether sulfone) Composite Membranes with Microporous Structures† [J]. Chem. J. Chinese Universities, 2019, 40(5): 1051. |
| [12] | LIN Zhouchen,HUANG Qiaoxi,LEI Ming. Fabrication and Electrocatalytic Performance of Graphene-fullerene Ammonium Iodide Composite Supported Pd Nanocatalyst for Ethanol Oxidation† [J]. Chem. J. Chinese Universities, 2019, 40(5): 1013. |
| [13] | LIU Jiaming,FU Kailin,ZHANG Ze,GUO Wei,PAN Mu. Ultra-low Pt Loading Cathodic Catalyst Layer Prepared on Textured Gas Diffusion Layer by Magnetron Sputtering Method for Hydrogen-oxygen Fuel Cells† [J]. Chem. J. Chinese Universities, 2019, 40(3): 542. |
| [14] | SHI Yue,MAO Qing,XIAO Cheng,JING Weiyun,ZHANG Xueyuan. Nonlinear Spectroscopy Analysis for Electrocatalytic Oxidation of Methanol on PtRu/C Surface† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2017. |
| [15] | ZHU Xingye,QIAN Huidong,JIANG Jingjing,YUE Zhouying,XU Jianfeng,ZOU Zhiqing,YANG Hui. Cross-linking of Imidazole-grafted Sulfonated Poly(ether ether ketone) as Proton Exchange Membranes for Direct Methanol Fuel Cells† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2046. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||