

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (12): 2315.doi: 10.7503/cjcu20160394
• Polymer Chemistry • Previous Articles
YAO Kuncheng1,2, NIE Huarong1, MA Yunsheng2, WANG Riguo3, WANG Xiaojian1, HE Aihua1,*( )
)
Received:2016-05-30
Online:2016-12-10
Published:2016-11-18
Contact:
HE Aihua
E-mail:aihuahe@iccas.ac.cn;ahhe@qust.edu.cn
Supported by:CLC Number:
TrendMD:
YAO Kuncheng, NIE Huarong, MA Yunsheng, WANG Riguo, WANG Xiaojian, HE Aihua. Crystallization Kinetics of trans-/cis-Polyisoprene Blends†[J]. Chem. J. Chinese Universities, 2016, 37(12): 2315.
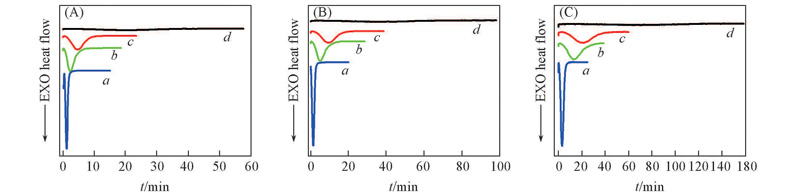
Fig.1 DSC curves of isothermal crystallization of TPI/CPI blends at crystallization temperatures of 20 ℃(A), 25 ℃(B) and 30 ℃(C) m(TPI)∶m(CPI): a. 100∶0; b. 80∶20; c. 50∶50; d. 30∶70.
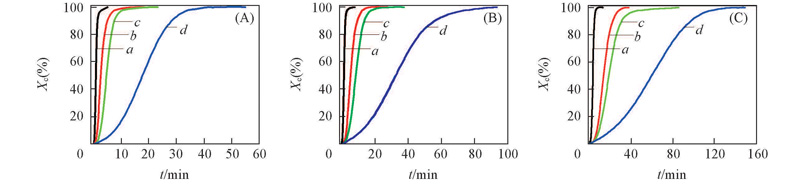
Fig.2 Relative crystallinity of TPI/CPI blends at crystallization temperatures of 20 ℃(A), 25 ℃(B) and 30 ℃(C)m(TPI)∶m(CPI): a. 100∶0; b. 80∶20; c. 50∶50; d. 30∶70.
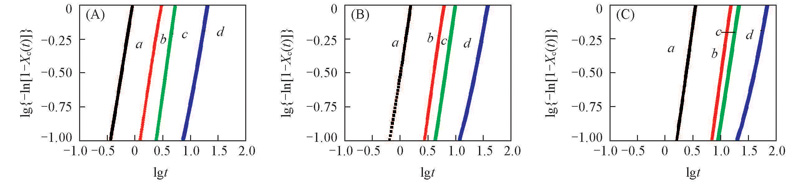
Fig.3 Plots of lg{-ln[1-X(t)]} vs. lgt for TPI/CPI blends at crystallization temperatures of 20 ℃(A), 25 ℃(B) and 30 ℃(C)m(TPI)∶m(CPI): a. 100∶0; b. 80∶20; c. 50∶50; d. 30∶70.
| Tc/℃ | m(TPI)∶m(CPI) | Δ | Δ | nb | Kb | |
|---|---|---|---|---|---|---|
| 20 | 100∶0 | 32.22 | 32.22 | 2.15 | 178.31 | 0.64 |
| 80∶20 | 32.19 | 40.24 | 2.60 | 6.03 | 2.56 | |
| 50∶50 | 24.37 | 48.74 | 2.92 | 0.79 | 4.66 | |
| 30∶70 | 14.27 | 47.57 | 2.29 | 0.09 | 17.68 | |
| 25 | 100∶0 | 39.29 | 39.29 | 2.60 | 31.44 | 1.36 |
| 80∶20 | 33.28 | 41.60 | 2.83 | 0.61 | 5.34 | |
| 50∶50 | 24.26 | 48.52 | 2.78 | 0.18 | 8.56 | |
| 30∶70 | 16.50 | 55.00 | 2.01 | 0.06 | 32.50 | |
| 30 | 100∶0 | 42.83 | 42.83 | 2.93 | 2.46 | 3.14 |
| 80∶20 | 31.60 | 39.50 | 2.89 | 0.04 | 13.44 | |
| 50∶50 | 22.52 | 45.04 | 2.66 | 0.03 | 18.54 | |
| 30∶70 | 18.75 | 62.50 | 1.89 | 0.03 | 59.64 |
Table 1 Crystallization parameters of TPI/CPI blends at different crystallization temperature
| Tc/℃ | m(TPI)∶m(CPI) | Δ | Δ | nb | Kb | |
|---|---|---|---|---|---|---|
| 20 | 100∶0 | 32.22 | 32.22 | 2.15 | 178.31 | 0.64 |
| 80∶20 | 32.19 | 40.24 | 2.60 | 6.03 | 2.56 | |
| 50∶50 | 24.37 | 48.74 | 2.92 | 0.79 | 4.66 | |
| 30∶70 | 14.27 | 47.57 | 2.29 | 0.09 | 17.68 | |
| 25 | 100∶0 | 39.29 | 39.29 | 2.60 | 31.44 | 1.36 |
| 80∶20 | 33.28 | 41.60 | 2.83 | 0.61 | 5.34 | |
| 50∶50 | 24.26 | 48.52 | 2.78 | 0.18 | 8.56 | |
| 30∶70 | 16.50 | 55.00 | 2.01 | 0.06 | 32.50 | |
| 30 | 100∶0 | 42.83 | 42.83 | 2.93 | 2.46 | 3.14 |
| 80∶20 | 31.60 | 39.50 | 2.89 | 0.04 | 13.44 | |
| 50∶50 | 22.52 | 45.04 | 2.66 | 0.03 | 18.54 | |
| 30∶70 | 18.75 | 62.50 | 1.89 | 0.03 | 59.64 |
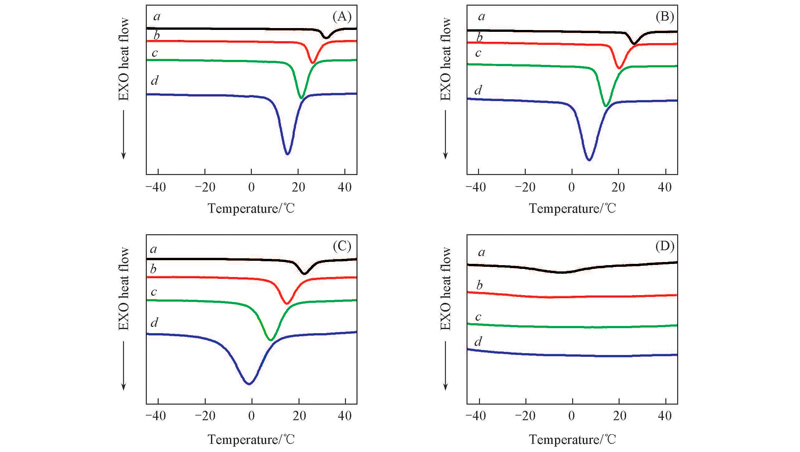
Fig.4 DSC thermograms with different cooling rates for neat TPI and TPI/CPI blends m(TPI)∶m(CPI): (A) 100∶0; (B) 80∶20; (C) 50∶50; (D) 30∶70. Cooling rate/(℃·min-1): a. 2; b. 5; c. 10; d. 20.
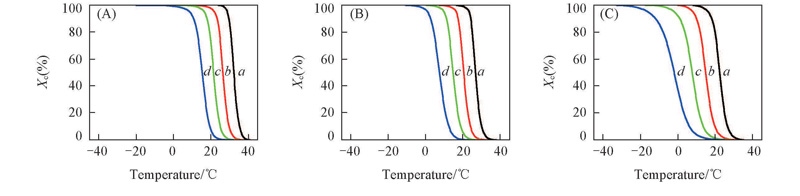
Fig.5 Plots of the relative crystallinity vs. crystallization temperature for TPI/CPI blends with different cooling ratesCooling rate/(℃·min-1): a. 2; b. 5; c. 10; d. 20.m(TPI)∶m(CPI): (A) 100∶0; (B) 80∶20; (C) 50∶50.
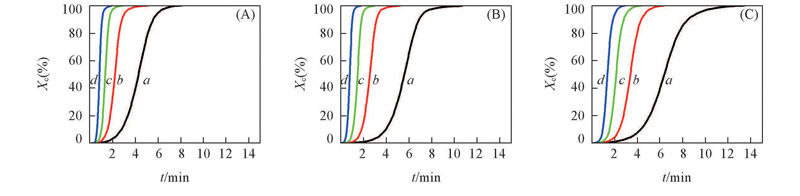
Fig.6 Plots of the relative crystallinity vs. crystallization time for TPI/CPI blends with different cooling ratesCooling rate/(℃·min-1): a. 2; b. 5; c.10; d. 20.m(TPI)∶m(CPI): (A) 100∶0; (B) 80∶20; (C) 50∶50.
| m(TPI)∶m(CPI) | Xc = 20% | Xc=40% | Xc=60% | Xc=80% | ||||
|---|---|---|---|---|---|---|---|---|
| F(T) | a | F(T) | a | F(T) | a | F(T) | a | |
| 100∶0 | 11.87 | 1.48 | 14.34 | 1.42 | 16.35 | 1.40 | 18.81 | 1.38 |
| 80∶20 | 12.05 | 1.17 | 14.40 | 1.18 | 16.36 | 1.19 | 18.73 | 1.21 |
| 50∶50 | 23.27 | 1.51 | 28.79 | 1.49 | 33.92 | 1.49 | 41.34 | 1.50 |
Table 2 Kinetic parameters for non-isothermal crystallization of TPI/CPI blends*
| m(TPI)∶m(CPI) | Xc = 20% | Xc=40% | Xc=60% | Xc=80% | ||||
|---|---|---|---|---|---|---|---|---|
| F(T) | a | F(T) | a | F(T) | a | F(T) | a | |
| 100∶0 | 11.87 | 1.48 | 14.34 | 1.42 | 16.35 | 1.40 | 18.81 | 1.38 |
| 80∶20 | 12.05 | 1.17 | 14.40 | 1.18 | 16.36 | 1.19 | 18.73 | 1.21 |
| 50∶50 | 23.27 | 1.51 | 28.79 | 1.49 | 33.92 | 1.49 | 41.34 | 1.50 |
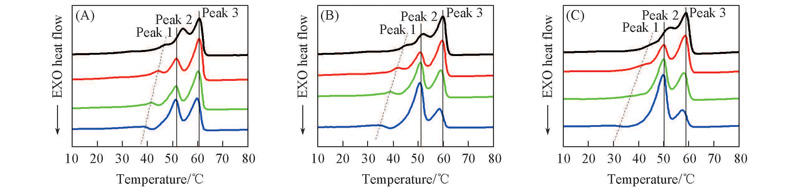
Fig.8 DSC heating curves with a heating rate of 10 ℃/min for TPI/CPI blendsHeating rate/(℃·min-1): a. 2; b. 5; c.10; d. 20. m(TPI)∶m(CPI): (A) 100∶0; (B) 80∶20; (C) 50∶50.
| Heating rate/(℃·min-1) | m(TPI)∶m(CPI) | Δ | Δ |
|---|---|---|---|
| 2 | 100∶0 | 49.17 | 49.17 |
| 80∶20 | 37.22 | 46.53 | |
| 50∶50 | 22.77 | 45.54 | |
| 5 | 100∶0 | 44.60 | 44.6 |
| 80∶20 | 34.35 | 42.94 | |
| 50∶50 | 22.31 | 44.62 | |
| 10 | 100∶0 | 42.16 | 42.16 |
| 80∶20 | 33.39 | 41.74 | |
| 50∶50 | 21.54 | 43.08 | |
| 20 | 100∶0 | 40.83 | 40.83 |
| 80∶20 | 33.30 | 41.63 | |
| 50∶50 | 21.38 | 42.76 |
Table 3 Values of melting enthalpy for non-isothermal crystallization of TPI/CPI blends
| Heating rate/(℃·min-1) | m(TPI)∶m(CPI) | Δ | Δ |
|---|---|---|---|
| 2 | 100∶0 | 49.17 | 49.17 |
| 80∶20 | 37.22 | 46.53 | |
| 50∶50 | 22.77 | 45.54 | |
| 5 | 100∶0 | 44.60 | 44.6 |
| 80∶20 | 34.35 | 42.94 | |
| 50∶50 | 22.31 | 44.62 | |
| 10 | 100∶0 | 42.16 | 42.16 |
| 80∶20 | 33.39 | 41.74 | |
| 50∶50 | 21.54 | 43.08 | |
| 20 | 100∶0 | 40.83 | 40.83 |
| 80∶20 | 33.30 | 41.63 | |
| 50∶50 | 21.38 | 42.76 |
| [1] | Bhowmick A. K., Kuo C. C., Manzur A., Arthur A. M., Intyre D. M., J. Macromol. Sci. Part B: Phys., 1986, 25(3), 283—309 |
| [2] | Carter A.J., Davies C. K. L., Thomas A. G., Paper Presented at the First Italian-Polish Seminar on Multicomponent Polemeric Systems, Plenum Press, NewYork, 1979 |
| [3] | Song J. S., Huang B. C., Yu D. S., J. Appl. Polym. Sci., 2001, 82(2), 81—89 |
| [4] | Bunn C. W., Proc. R. Soc. Lond. Ser. A, 1942, 180(980), 40—66 |
| [5] | Mandelkern L., Quinn F. A., Roberts D. E., J. Am. Chem. Soc., 1956, 78(5), 926—932 |
| [6] | Fischer E., Henderson J. F., J. Polym. Sci. Part A, 1967, 5(2), 377—390 |
| [7] | Candia F. D., Romano G., Taglialatela A., Vittoria V., Polym. Bull., 1981, 4(4), 233—240 |
| [8] | Baboo M., Dixit M., Sharma K., Saxena N. S., Polym. Bull., 2011, 66(5), 661—672 |
| [9] | Cooper W., Vaughan G., Polymer, 1962, 4, 329—340 |
| [10] | Yao K. C., Nie H. Y., Liang Y. R., Qiu D., He A. H., Polymer, 2015, 80, 259—264 |
| [11] | Avrami M., J. Chem. Phys., 1939, 7(12), 1103—1112 |
| [12] | Liu T. X., Mo Z. S., Wang S. G., Zhang H. F., Polym. Eng. Sci., 1997, 37(3), 568—575 |
| [13] | Liu L., Wang B. X., Wang Y. R., China Synthetic Rubber Industry, 2012, 35(6), 433—436 |
| (刘莉, 王炳昕, 王韵然. 合成橡胶工业,2012, 35(6), 433—436) | |
| [14] | Lovering E. G., Wooden D. C., J. Polym. Sci. Part A-2, 1969, 7(10), 1639—1649 |
| [15] | Ratri P. J., Tashiro K., Iguchi M., Polymer, 2012, 53, 3548—3558 |
| [16] | Takahashi Y., Sato T., Tadokoro H., J. Polym. Sci. Part B, 1973, 11(2), 233—248 |
| [1] | YAN Zhixuan, MA Ji, QU Jinlei, LIU Li, SUN Chong, LIU Jiwen, LIU Guangye, SUN Lishui, HE Lixia. Synthesis and Application of Modified Low Molecular Weight Polyisoprene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220066. |
| [2] | WANG Meilin, LIU Yudong, LIU Xiaoli, LI Zhiying, LIU Fengqi. Nonisothermal Crystallization Kinetics of ABS/PET/PETG Alloy† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1290. |
| [3] | REN Huicheng,WU Yingfei,LIU Dandan,NIE Huarong,HE Aihua. Crystallization, Nucleation and Kinetics of TPI in SSBR/TPI Blends† [J]. Chem. J. Chinese Universities, 2018, 39(5): 1091. |
| [4] | NIU Qingtao,ZOU Chen,WANG Riguo,LI Lange,HE Aihua. Fractionation and Fraction Characterization of trans-Polyisoprene Rubber Alloys within Reactor† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2312. |
| [5] | WANG Hao, ZHANG Jianping, MA Yunsheng, WANG Riguo, HE Aihua. Modification of SSBR/BR with Trans-1,4-Polyisoprene Alloy Rubber for High Performance Passenger Car Radical Tire Tread Stock† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2095. |
| [6] | SI Pengfei, LUO Faliang, HAI Mei. Intermolecular Interactions and Crystallization and Melting Behavior of Poly(L-lactic acid)/4,4'-Thiobis Phenol Blends† [J]. Chem. J. Chinese Universities, 2015, 36(1): 188. |
| [7] | GUO Lai-Hui, FANG Xing-Zhong*, WANG Gui-Bin, WU Zhong-Wen. Isothermal Crystallization Kinetics of Thermoplastic Polyimide and Poly(ether ether ketone) Blends [J]. Chem. J. Chinese Universities, 2011, 32(12): 2908. |
| [8] | ZHU Ming, LI Ya-Peng, TANG Jun, WANG Shu-Wei, WANG Jing-Yuan*. Nonisothermal Crystallization Kinetics of P(BHB-CL) [J]. Chem. J. Chinese Universities, 2010, 31(3): 607. |
| [9] | REN Jie*, GUI Bao-Zhu, REN Tian-Bin, YANG Jun, GU Ming-Hao. Non-isothermal Crystallization Behavior of PLLA-PEG Copolymer [J]. Chem. J. Chinese Universities, 2007, 28(10): 2006. |
| [10] | LI Gui-Juan1,2*; XU Xiao-Duo1; XU Xue-Li1; YU Bao-Jie1; LI Yi1; ZHOU En-Le2. Sudies on Non-isothermal Melt Crystallization Kinetics in PET/PEN/DBS Blends [J]. Chem. J. Chinese Universities, 2006, 27(6): 1173. |
| [11] |
YANG Jun-Liang; ZHAO Ting; CUI Ji-Jun; LIU Lei-Jing; ZHOU Yun-Chun; LI Gao*; ZHOU En-Le; CHEN Xue-Si. Nonisothermal Crystallization Behavior of Poly(L-lactide)-Poly(ethylene glycol) Diblock Copolymer [J]. Chem. J. Chinese Universities, 2006, 27(6): 1162. |
| [12] | CHEN Qing, FAN Yu-Run, LI Wen-Chun, Zheng-Qiang. Rheological Properties of Liquid-solid Transition in Isothermal Crystallization for High-density Polyethylene [J]. Chem. J. Chinese Universities, 2006, 27(2): 365. |
| [13] | SUN Yuan-Bi, Xu-Jun, Xu-Yong-Xiang, Yan-Li-Tang, Guo-Bao-Hua. Synthesis and Characterization of Biodegradable Poly(butylene succinate-co-butylene methyl succinate) [J]. Chem. J. Chinese Universities, 2006, 27(2): 360. |
| [14] | SHANG Guan-Yong-Gang, ZHENG Qiang, BANG Mao, WANG Hui-Jun, ZHANG Ming-Qiu. Crystalline Structure of Polypropylene Catalloys and Related Influence Factors [J]. Chem. J. Chinese Universities, 2005, 26(12): 2386. |
| [15] | SHANGGUAN Yong-Gang, ZHENG Qiang, PENG Mao, WANG Hui-Jun, ZHANG Ming-Qiu. Crystalline Structure of Polypropylene Catalloys and Related Influence Factors [J]. Chem. J. Chinese Universities, 2005, 26(12): 2386. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||