

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (10): 2009.doi: 10.7503/cjcu20150207
• Physical Chemistry • Previous Articles Next Articles
LIANG Weiping, ZHAO Xiaohui, AN Dong, XU Qian, YE Zhiwen*( )
)
Received:2015-03-18
Online:2015-10-10
Published:2015-09-18
Contact:
YE Zhiwen
E-mail:yezw@njust.edu.cn
CLC Number:
TrendMD:
LIANG Weiping, ZHAO Xiaohui, AN Dong, XU Qian, YE Zhiwen. Synthesis and Surface Properties of Siloxane Gemini Imidazolium Surfactant†[J]. Chem. J. Chinese Universities, 2015, 36(10): 2009.
| Surfactant | cmc/(mmol·L-1) | γcmc/(mN·m-1) | πcmc/(mN·m-1) | Гmax/(μmol·m-2) | pc20 | Amin /nm2 |
|---|---|---|---|---|---|---|
| [Si4-4-Si4im]Cl2 | 0.54 | 18.6 | 53.4 | 0.704 | 6.04 | 2.36 |
| [C14-4-C14im]Cl2 | 0.27 | 39.5 | 32.5 | 0.811 | 4.47 | 2.05 |
| [Si4mim]Cl[ | 7.4 | 20.4 | 52.1 | 0.84 | 5.43 | 1.98 |
Table 1 Adsorption parameters of the surfactants in aqueous solution at 298.15 K
| Surfactant | cmc/(mmol·L-1) | γcmc/(mN·m-1) | πcmc/(mN·m-1) | Гmax/(μmol·m-2) | pc20 | Amin /nm2 |
|---|---|---|---|---|---|---|
| [Si4-4-Si4im]Cl2 | 0.54 | 18.6 | 53.4 | 0.704 | 6.04 | 2.36 |
| [C14-4-C14im]Cl2 | 0.27 | 39.5 | 32.5 | 0.811 | 4.47 | 2.05 |
| [Si4mim]Cl[ | 7.4 | 20.4 | 52.1 | 0.84 | 5.43 | 1.98 |
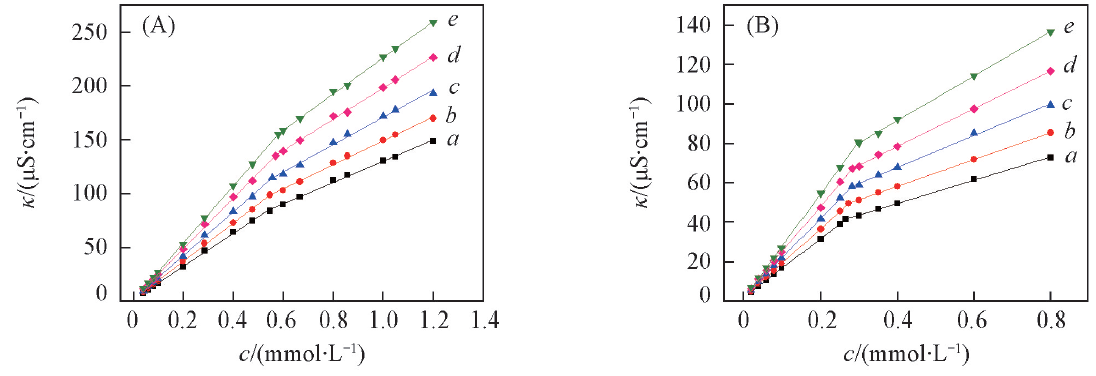
Fig.3 Specific conductivity as a function of concentration at different temperatures(A) [Si4-4-Si4im]Cl2; (B) [C14-4-C14im]Cl2. Temperature/℃: a. 15; b. 20; c. 25; d. 30; e. 35.
| Surfactant | T/K | cmc/(mmol·L-1) | β | Δ | Δ | TΔ |
|---|---|---|---|---|---|---|
| [Si4-4-Si4im]Cl2 | 288.15 | 0.533 | 0.378 | -24.30 | -2.690 | 21.610 |
| 293.15 | 0.547 | 0.378 | -24.67 | -2.785 | 21.885 | |
| 298.15 | 0.557 | 0.373 | -24.89 | -2.862 | 22.028 | |
| 303.15 | 0.570 | 0.369 | -25.16 | -2.949 | 22.211 | |
| 308.15 | 0.580 | 0.358 | -25.21 | -3.008 | 22.202 | |
| [C14-4-C14im]Cl2 | 288.15 | 0.265 | 0.609 | -32.56 | -4.571 | 27.989 |
| 293.15 | 0.273 | 0.614 | -33.17 | -4.750 | 28.420 | |
| 298.15 | 0.281 | 0.602 | -33.31 | -4.862 | 28.448 | |
| 303.15 | 0.282 | 0.588 | -33.42 | -4.963 | 28.457 | |
| 308.15 | 0.297 | 0.588 | -33.82 | -5.127 | 28.693 | |
| [Si4mim]Cl[ | 288.15 | 6.6 | 0.32 | -28.64 | -40.48 | -11.84 |
| 293.15 | 6.9 | 0.30 | -28.42 | -44.39 | -15.97 | |
| 298.15 | 7.5 | 0.27 | -28.11 | -48.17 | -20.06 | |
| 303.15 | 8.2 | 0.25 | -27.71 | -51.82 | -24.11 | |
| 308.15 | 9.0 | 0.22 | -27.32 | -55.35 | -28.03 |
Table 2 Influence of temperature on the parameters of micellization for the surfactants in aqueous solution
| Surfactant | T/K | cmc/(mmol·L-1) | β | Δ | Δ | TΔ |
|---|---|---|---|---|---|---|
| [Si4-4-Si4im]Cl2 | 288.15 | 0.533 | 0.378 | -24.30 | -2.690 | 21.610 |
| 293.15 | 0.547 | 0.378 | -24.67 | -2.785 | 21.885 | |
| 298.15 | 0.557 | 0.373 | -24.89 | -2.862 | 22.028 | |
| 303.15 | 0.570 | 0.369 | -25.16 | -2.949 | 22.211 | |
| 308.15 | 0.580 | 0.358 | -25.21 | -3.008 | 22.202 | |
| [C14-4-C14im]Cl2 | 288.15 | 0.265 | 0.609 | -32.56 | -4.571 | 27.989 |
| 293.15 | 0.273 | 0.614 | -33.17 | -4.750 | 28.420 | |
| 298.15 | 0.281 | 0.602 | -33.31 | -4.862 | 28.448 | |
| 303.15 | 0.282 | 0.588 | -33.42 | -4.963 | 28.457 | |
| 308.15 | 0.297 | 0.588 | -33.82 | -5.127 | 28.693 | |
| [Si4mim]Cl[ | 288.15 | 6.6 | 0.32 | -28.64 | -40.48 | -11.84 |
| 293.15 | 6.9 | 0.30 | -28.42 | -44.39 | -15.97 | |
| 298.15 | 7.5 | 0.27 | -28.11 | -48.17 | -20.06 | |
| 303.15 | 8.2 | 0.25 | -27.71 | -51.82 | -24.11 | |
| 308.15 | 9.0 | 0.22 | -27.32 | -55.35 | -28.03 |
| [1] | Liu J. G., China Surfactant Soap and Detergent Industries, 2008, 5, 68—73 |
| (刘节根. 中国洗涤用品工业, 2008, 5, 68—73) | |
| [2] | Shi C.L., Synthesis of a Novel Class of Organosilicon Quaternary Ammonium Gemini Surfactants, Shandong Polytechnic University, Jinan, 2012 |
| (石翠磊. 有机硅双季铵盐的合成, 济南: 山东轻工业学院, 2012) | |
| [3] | Wu L.Y., Synthesis and Performances of Trisiloxane Surfactant, Zhejiang University, Hangzhou, 2007 |
| (吴鲁燕. 三硅氧烷表面活性剂的合成及性能研究, 杭州: 浙江大学, 2007) | |
| [4] | Cao Y., Du Z. P., Wang G. Y., Textile Auxiliaries, 2012, 29(4), 12—16 |
| (曹洋, 杜志平, 王国永. 印染助剂,2012, 29(4), 12—16) | |
| [5] | Tan J. L., Ma D. P., Feng S. Y., Zhang C. Q., Colloids and Surfaces A: Physicochem. Eng. Aspects, 2013, 417 , 146—153 |
| [6] | Tan J. L., Zhao P. J., Ma D. P., Feng S. Y., Zhang C. Q., Colloids Polym. Sci., 2013, 291, 1487—1494 |
| [7] | Liu H. Y., Gu D. M., Liu G. Y., Zhao X. L., Chen C., Xu K. M., Chem.J. Chinese Universities, 2013, 34(2), 401—407 |
| (刘海燕, 顾大明, 刘国宇, 赵秀丽, 陈成, 徐克明. 高等学校化学学报,2013, 34(2), 401—407) | |
| [8] | Gonsior N., Mohr F., Ritter H., Beilstein Journal of Organic Chemistry, 2012, 8, 390—397 |
| [9] | Amarasekata S. A., Shanbhag P., Polym. Bull., 2011, 67, 623—629 |
| [10] | Du J., Tang L., Journal of Jilin Normal University, 2009, 4, 119—120 |
| (杜娟, 唐璐. 吉林师范大学学报, 2009, 4, 119—120) | |
| [11] | Menger M.F., Lu H.,Chem. Commun., 2006, 3235—3237 |
| [12] | Christoffers J., Oertling H., Fischer P., Frey W., Tetrahedron, 2003, 59, 3769—3778 |
| [13] | Liu G. Y., Gu D. M., Liu H. Y., Gu S., Journal of Harbin Institute of Technology, 2013, 45(7), 72—78 |
| (刘国宇, 顾大明, 刘海燕, 顾硕. 哈尔滨工业大学学报,2013, 45(7), 72—78) | |
| [14] | Wang D., Synthesis and the Study on Phase Behavior of Alkylimidazolinium Gemini Surfactant, Shandong Normal University, Jinan, 2013 |
| (王丹. 烷基咪唑Gemini型表面活性剂的合成及相行为研究, 济南: 山东师范大学, 2013) | |
| [15] | Suzuki Y., Wakatsuki J., Tsubaki M., Sato M., Tetrahedron, 2013, 69, 9690—9700 |
| [16] | Khlebnikov V., Heckenroth M., Albrecht M., Albrecht M., Dalton Trans., 2013, 42, 4197—4207 |
| [17] | Vogt M., Pons V., Heinekey D. M., Organometallics, 2005, 24, 1832—1836 |
| [18] | Ao M. Q., Xu G. Y., Zhu Y. Y., Bai Y., Journal of Colloid and Interface Science, 2008, 326, 490—495 |
| [19] | Bhattacharya S., Haldar J., Langmuir, 2004, 20(19), 7940—7947 |
| [20] | Nusselder J. J. H., Engberts J. B. N., J. Colloid Interf. Sci., 1992, 148(2), 353—361 |
| [21] | Hong Y., Preparation and Performance of Quaternary Ammonium Gemini Surfactant, Shanxi University of Science and Technology, Xi’an, 2014 |
| (洪玉. 季铵盐型双子表面活性剂的合成及其性能研究, 西安: 陕西科技大学, 2014) |
| [1] | ZHENG Zirui, LI Zilu, ZHAO Kefei, WU Tianyue, ZHANG Chenhui, GAO Yuxia, DU Fengpei. Interfacial Behaviors of Bio-based Surfactant Escin [J]. Chem. J. Chinese Universities, 2021, 42(10): 3107. |
| [2] | HUANG Hailong, LI Hongyu, ZHANG Lei, REN Tianrui. Surface and Interfacial Properties of Polyoxyethylene Ether-type Surfactants† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1330. |
| [3] | LIU Hai-Yan, GU Da-Ming, LIU Guo-Yu, ZHAO Xiu-Li, CHEN Cheng, XU Ke-Ming. Synthesis and Surface Properties of Novel Gemini Imidazolium Surfactants [J]. Chem. J. Chinese Universities, 2013, 34(2): 401. |
| [4] | LIANG Ya-Qin, HU Zhi-Yong, CAO Duan-Lin, LIANG Dong. Surface Activity Properties of Chiral L-Lysine Based Gemini Surfactants [J]. Chem. J. Chinese Universities, 2013, 34(12): 2783. |
| [5] | JIN Xing-Hui, HU Bing-Cheng, JIA Huan-Qing, LIU Zu-Liang, LÜ Chun-Xu. Synthesis and Theoretical Studies of 1,2,3-Triaminoguanidinium Dinitroguanidinate Salt [J]. Chem. J. Chinese Universities, 2013, 34(12): 2821. |
| [6] | YANG Bai-Qin, LU Guo-Chao, XING Hang, ZHOU Hong-Tao, GAO An-Tao, XIAO Jin-Xin. Interactions Between Sodium p-Perfluorononenyloxy Benzene Sulfonate and Alkyl Quaternary Ammonium Salts [J]. Chem. J. Chinese Universities, 2012, 33(12): 2765. |
| [7] | ZENG Zhi-Yong, LI Xiao-Sen*. Hydrate Formation Phase Equilibrium Model in the Porous Media Based on PC\|SAFT Equation of State [J]. Chem. J. Chinese Universities, 2011, 32(4): 908. |
| [8] | YANG Fang, LI Gang*, ZHANG Song-Mei, QI Jian, LIU Rong, XU Nian. Synthesis and Surface Properties of Nonylphenol Polyoxyethylene Dimeric and Trimeric Nonionic Surfactants [J]. Chem. J. Chinese Universities, 2010, 31(7): 1465. |
| [9] | ZENG You*, ZHAO Long, LIU Peng-Fei, DU Jin-Hong, LIU Chang. Thermal and Electrical Conductivities of the Graphitized Carbon Nanotube/poly(methyl methacrylate) Composites Based on Weak Nanotube-polymer Interactions [J]. Chem. J. Chinese Universities, 2010, 31(6): 1263. |
| [10] | BI Ya-Dong, XU Heng-Yong*, LI Wen-Zhao. Electrical Conductivity Study of Pt/Ce0.6Zr0.4O2 Catalyst in Water-gas Shift Reaction [J]. Chem. J. Chinese Universities, 2009, 30(5): 945. |
| [11] | WU Qiu-Hua, WEI Tian-Zhu, LIANG Fang, SONG Xi-Ming, HAN Guang-Xi, ZHANG Guo-Lin*. Synthesis and Micellization of Polyacrylamide/Poly(γ-benzyl-L-glutamate) Graft Copolymer [J]. Chem. J. Chinese Universities, 2008, 29(8): 1650. |
| [12] | WANG Xiao-Chun1, ZHANG Lei1, GONG Qing-Tao1, WANG Lin1, ZHANG Lu1, LI Zhen-Quan2, ZHAO Sui1*, YU Jia-Yong1. Foaming Properties and Dynamic Surface Tension of Sodium Alkylbenzenesulfonates with Different Structures [J]. Chem. J. Chinese Universities, 2007, 28(11): 2118. |
| [13] | XIN Zhi-Rong1,2, ZHAO Yu-Xia2, HOU Wan-Guo1, YIN Li-Gang3, QIU Zhao-Ming2, LIU Xiao-Li2, LIU Chan2, YIN Jing-Hua3*. Preparation and Chracaterization of Novel Graft Copolymers of LLDPE [J]. Chem. J. Chinese Universities, 2007, 28(10): 1990. |
| [14] | LI Qian, YUE Qin-Yan*, GAO Bao-Yu, LIU Li-Li. Kinetics of Adsorption of Disperse Dyes at Cationic Bentonites [J]. Chem. J. Chinese Universities, 2006, 27(6): 1113. |
| [15] | ZHANG Qing-Guo1,2, GUANG Wei2, TONG Jing2, JIN Zhen-Xing 1*. Studies on Properties of Ionic Liquid EMIFeCl4 Based on Transition Metal [J]. Chem. J. Chinese Universities, 2006, 27(5): 925. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||