

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (6): 1117.doi: 10.7503/cjcu20141110
• Organic Chemistry • Previous Articles Next Articles
LIU Yali, LIU De’e, DU Manfei, CAO Chenxi, CHEN Zhanguo*( )
)
Received:2014-12-18
Online:2015-06-10
Published:2015-04-28
Contact:
CHEN Zhanguo
E-mail:chzhg@snnu.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Yali, LIU De’e, DU Manfei, CAO Chenxi, CHEN Zhanguo. High Regioselective Aminobromination of β-Nitrostyrene Derivatives with 1,3-Dibromo-5,5-dimethyl Hydantoin†[J]. Chem. J. Chinese Universities, 2015, 36(6): 1117.
| Compd. | m.p.(ref.)/℃ | Compd. | m.p.(ref.)/℃ |
|---|---|---|---|
| 1a | 55.1—56.3(55—56[ | 11a | 145—146(128—131 [ |
| 2a | 100.1—102.1(102—102.5 [ | 12a | 61.5—61.7(60—61 [ |
| 3a | 84.4—86.0(85.5—87.5 [ | 13a | 54.0—54.4(55[ |
| 4a | 120.2—120.6(125.5—126.5 [ | 14a | 42.0—42.3(43—44.5[ |
| 5a | 98—99(98—99[ | 15a | 96.4—97.3(96—97[ |
| 6a | 98.6—99.1(101—102.5[ | 16a | 64.0—65.1(66[ |
| 7a | 108.5—110.4(112—113[ | 17a | 88.6—89.9(86—87[ |
| 8a | 149.3—150.6(151—153 [ | 18a | 89.3—91.3(93—95[ |
| 9a | 201.2—203.1(203—204 [ | 19a | 113.8—114.1(114—115[ |
| 10a | 123.1—124.3(124.5—126 [ | 20a | 56.1—56.3(55—55.5[ |
Table 1 Melting points of β-nitrostyrene derivatives
| Compd. | m.p.(ref.)/℃ | Compd. | m.p.(ref.)/℃ |
|---|---|---|---|
| 1a | 55.1—56.3(55—56[ | 11a | 145—146(128—131 [ |
| 2a | 100.1—102.1(102—102.5 [ | 12a | 61.5—61.7(60—61 [ |
| 3a | 84.4—86.0(85.5—87.5 [ | 13a | 54.0—54.4(55[ |
| 4a | 120.2—120.6(125.5—126.5 [ | 14a | 42.0—42.3(43—44.5[ |
| 5a | 98—99(98—99[ | 15a | 96.4—97.3(96—97[ |
| 6a | 98.6—99.1(101—102.5[ | 16a | 64.0—65.1(66[ |
| 7a | 108.5—110.4(112—113[ | 17a | 88.6—89.9(86—87[ |
| 8a | 149.3—150.6(151—153 [ | 18a | 89.3—91.3(93—95[ |
| 9a | 201.2—203.1(203—204 [ | 19a | 113.8—114.1(114—115[ |
| 10a | 123.1—124.3(124.5—126 [ | 20a | 56.1—56.3(55—55.5[ |
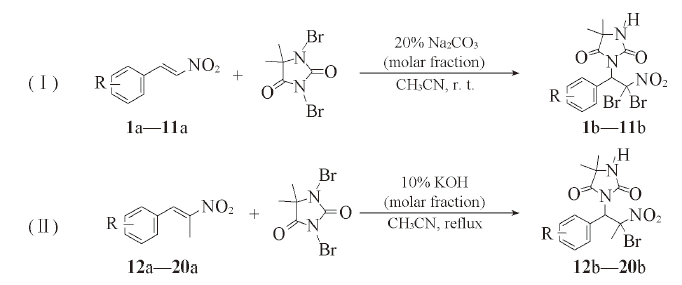
Scheme 1 Aminobromination of β-nitrostyrene derivatives with DBDMH1a, 1b, 12a, 12b: R=H; 2a, 2b, 13a, 13b: R=CH3; 3a, 3b, 14a, 14b: R=4-OMe; 4a, 4b: R=3,4,5-(OMe)3; 5a, 5b, 15a, 15b: R=2-Br-4,5-(OMe)2; 6a, 6b, 16a, 16b: R=F; 7a, 7b, 17a, 17b: R=Cl; 8a, 8b, 18a, 18b: R=Br; 9a, 9b, 19a, 19b: R=4-NO2; 10a, 10b, 20a, 20b: R=3-NO2; 11a, 11b: R=—CHCHCHCH—
| Compd. | Appearance | Yield*(%) | m. p./℃ | HRMS [M+Na]+(calcd.), m/z |
|---|---|---|---|---|
| 1b | White solid | 94 | 167—169 | 457.9151(457.9150) |
| 2b | White solid | 89 | 147—149 | 471.9295(471.9307) |
| 3b | White solid | 85 | 142—144 | 487.9246(487.9256) |
| 4b | White solid | 45 | 169—171 | 547.9466(547.9467) |
| 5b | Pale yellow solid | 65 | 200—202 | 595.8471(595.8466) |
| 6b | Pale yellow solid | 95 | 166.8—169.0 | 475.9046(475.9056) |
| 7b | White solid | 95 | 180—182 | 491.8763(491.8760) |
| 8b | White solid | 94 | 155—157 | 535.8259(535.8255) |
| 9b | White solid | 97 | 185—187 | 502.8991(502.9001) |
| 10b | White solid | 96 | 164—165 | 502.8988(502.9001) |
| 11b | White solid | 90 | 164—165 | 507.9308(507.9307) |
| 12b | White solid | 90 | 164.4—166.7 | 392.0214(392.0222) |
| 13b | White solid | 78 | 166.1—168.1 | 406.0374(406.0378) |
| 14b | White solid | 76 | 168.7—170.4 | 422.0327(422.0328) |
| 15b | Yellow solid | 84 | 156.1—157.5 | 531.9513(531.9518) |
| 16b | Pale yellow solid | 95 | 164.8—165.7 | 410.0123(410.0128) |
| 17b | Yellow solid | 94 | 199.5—201.4 | 425.9826(425.9832) |
| 18b | White solid | 94 | 177.1—178.9 | 471.9302(471.9307) |
| 19b | White solid | 95 | 148.6—149.9 | 437.0077(437.0073) |
| 20b | White solid | 93 | 175.5—176.3 | 437.0067(437.0073) |
Table 2 Appearance, yields, melting points and HRMS data for compounds 1b—20b
| Compd. | Appearance | Yield*(%) | m. p./℃ | HRMS [M+Na]+(calcd.), m/z |
|---|---|---|---|---|
| 1b | White solid | 94 | 167—169 | 457.9151(457.9150) |
| 2b | White solid | 89 | 147—149 | 471.9295(471.9307) |
| 3b | White solid | 85 | 142—144 | 487.9246(487.9256) |
| 4b | White solid | 45 | 169—171 | 547.9466(547.9467) |
| 5b | Pale yellow solid | 65 | 200—202 | 595.8471(595.8466) |
| 6b | Pale yellow solid | 95 | 166.8—169.0 | 475.9046(475.9056) |
| 7b | White solid | 95 | 180—182 | 491.8763(491.8760) |
| 8b | White solid | 94 | 155—157 | 535.8259(535.8255) |
| 9b | White solid | 97 | 185—187 | 502.8991(502.9001) |
| 10b | White solid | 96 | 164—165 | 502.8988(502.9001) |
| 11b | White solid | 90 | 164—165 | 507.9308(507.9307) |
| 12b | White solid | 90 | 164.4—166.7 | 392.0214(392.0222) |
| 13b | White solid | 78 | 166.1—168.1 | 406.0374(406.0378) |
| 14b | White solid | 76 | 168.7—170.4 | 422.0327(422.0328) |
| 15b | Yellow solid | 84 | 156.1—157.5 | 531.9513(531.9518) |
| 16b | Pale yellow solid | 95 | 164.8—165.7 | 410.0123(410.0128) |
| 17b | Yellow solid | 94 | 199.5—201.4 | 425.9826(425.9832) |
| 18b | White solid | 94 | 177.1—178.9 | 471.9302(471.9307) |
| 19b | White solid | 95 | 148.6—149.9 | 437.0077(437.0073) |
| 20b | White solid | 93 | 175.5—176.3 | 437.0067(437.0073) |
| Compd. | 1H NMR(300 MHz, CDCl3), δ | 13C NMR(75 MHz, CDCl3), δ |
|---|---|---|
| 1b | 7.74(dd, J=7.7, 1.5 Hz, 2H, ArH), 7.45—7.33(m, 3H, ArH), 6.80(s, 1H, NH), 6.46(s, 1H ,CH), 1.49(s, 3H ,CH3), 1.43(s, 3H, CH3) | 176.12, 154.95, 131.95, 130.55, 129.97, 128.60, 89.90, 66.11, 58.56, 25.19, 24.88 |
| 2b | 7.62(d, J=8.2 Hz, 2H, ArH), 7.17(d, J=8.1 Hz, 2H, ArH), 6.94(s, 1H , NH), 6.42(s, 1H, CH), 2.34(s, 3H, ArCH3), 1.48(s, 3H , CH3), 1.42(s, 3H, CH3) | 176.15, 155.01, 140.06, 130.45, 129.24, 128.87, 90.18, 65.96, 58.48, 25.11, 24.79, 21.20 |
| 3b | 7.70(d, J=8.9 Hz, 2H, ArH), 6.88(d, J=8.9 Hz, 2H , ArH), 6.60(s, 1H, NH), 6.42(s, 1H, CH), 3.81(s, 3H, OCH3), 1.49(s, 3H, CH3), 1.43(s, 3H, CH3) | 176.07, 160.67, 154.90, 132.30, 123.62, 113.84, 90.55, 65.86, 58.47, 55.26, 25.20, 24.89 |
| 4b | 7.07(s, 2H, ArH), 6.50(s, 1H, NH), 6.41(s, 1H, CH), 3.86(d, J=7.2 Hz, 9H, 3OCH3), 1.45(s, 3H, CH3) | 176.14, 154.72, 152.89, 139.31, 126.70, 108.54, 90.31, 66.42, 60.84, 58.43, 56.35, 25.24, 24.94 |
| 5b | 8.06(s, 1H, ArH), 7.04(s, 1H, NH), 6.76(s, 1H, NH), 6.74(s, 1H, CH), 3.91(s, 3H, OCH3), 3.88(s, 3H, OCH3), 1.47(s, 3H, CH3), 1.39(s, 3H, CH3) | 175.98, 154.58, 150.33, 147.37, 122.62,116.50, 115.21, 114.83, 90.34, 63.98, 58.43, 56.09, 56.08, 25.16, 24.84 |
| 6b | 7.79(d, J=3.7 Hz, 2H, ArH), 7.07(d, J=17.3 Hz, 2H ,ArH), 6.45(s, 1H, NH), 6.31(s, 1H, CH), 1.49(s, 3H, CH3), 1.42(s, 3H, CH3) | 176.07, 154.87, 131.95, 130.56, 129.95, 128.58, 89.89, 66.12, 58.53, 25.20, 24.90 |
| 7b | 7.72(d, J=8.4 Hz, 2H, ArH), 7.36(d, J=8.5 Hz, 2H, ArH), 6.52(s, 1H, NH), 6.41(s, 1H, CH), 1.46(d, J=22.2 Hz, 6H, 2CH3) | 175.93, 154.58, 136.28, 132.13, 130.28, 128.83, 89.45, 65.52, 58.61, 25.20, 24.92 |
| 8b | 7.65(d, J=8.5 Hz, 2H, ArH), 7.52(d, J=8.5 Hz, 2H, ArH), 6.68(s, 1H, NH), 6.39(s, 1H, CH), 1.49(s, 3H, CH3), 1.43(s, 3H, CH3) | 175.96, 154.69, 132.34, 131.81, 130.83, 124.62, 89.33, 65.60, 58.63, 25.17, 24.89 |
| 9b | 8.25(d, J=8.9 Hz, 2H, ArH), 7.98(d, J=8.8 Hz, 2H, ArH), 6.51(s, 1H, NH), 5.94(s, 1H, CH), 1.51(s, 3H, CH3), 1.45(s, 3H, CH3) | 175.70, 153.89, 148.63, 138.55, 131.81, 123.60 88.24, 65.14, 58.77, 25.24, 25.02 |
| 10b | 8.75(d, J=1.5 Hz, 1H , ArH), 8.30(d, J=9.6 Hz, 1H, ArH), 8.14(d, J=7.9 Hz, 1H, ArH), 7.62(t, J=8.1 Hz, 1H, ArH), 6.69(s, 1H, NH), 6.50(s, 1H, CH), 1.52(s, 3H, CH3), 1.47(s, 3H, CH3) | 175.96, 154.36, 148.03, 136.72, 133.66, 129.60, 126.00, 124.89, 88.53, 65.19, 58.87, 25.12, 24.92 |
| 11b | 8.55(d, J=7.5 Hz, 1H, ArH), 7.99(d, J=8.6 Hz, 1H, ArH), 7.89(dd, J=14.0, 8.1 Hz, 2H, ArH), 7.53(dd, J=22.9, 15.0, 7.4 Hz, 3H, ArH), 7.22(s, 1H, NH), 6.17(s, 1H,CH), 1.42(s, 3H, CH3), 1.27(s, 3H, CH3) | 175.84, 154.48, 133.87, 131.42, 130.59, 129.53, 128.57, 127.57, 126.50, 125.94, 124.43, 121.60, 89.73, 60.01, 58.47, 25.20, 24.79 |
| 12b | 7.77(dd, J=7.3, 2.1 Hz, 2H, ArH), 7.45—7.34(m, 3H, ArH), 6.69(s, 1H, NH), 6.20(s, 1H, CH), 2.59(s, 3H, CH3), 1.39(d, J=3.5 Hz, 6H, 2×CH3) | 176.99, 155.15, 132.96, 130.74, 129.54, 128.68, 95.75, 63.97, 58.35, 29.12, 24.93, 24.72 |
| 13b | 7.65(d, J=8.2 Hz, 2H, ArH), 7.20(d, J=8.0 Hz, 2H, ArH), 6.51(s, 1H, NH), 6.17(s, 1H, CH), 2.59(s, 3H, CH3), 2.37(ArCH3), 1.38(d, J=6.2 Hz, 6H, 2×CH3) | 176.84, 139.39, 130.54, 129.96, 129.16, 96.02, 63.77, 58.31, 29.11, 24.98, 24.80 |
| 14b | 7.71(d, J=8.8 Hz, 2H, ArH), 6.91(d, J=8.9 Hz, 2H, ArH), 6.48(s, 1H, NH), 6.15(s, 1H, CH), 3.82(s, 3H, OCH3), 2.58(s,3H, CH3), 1.38(d, J=4.5 Hz, 6H, 2×CH3) | 176.87, 160.38, 155.06, 132.31, 124.80, 113.98, 96.33, 63.55, 58.32, 55.29, 29.04, 24.90 |
| 15b | 7.91(s, 1H, ArH), 7.06(s, 1H, ArH), 6.76(s, 1H, NH), 6.65(s, 1H, CH), 3.88(s, 3H, OCH3), 3.87(s, 3H, OCH3), 2.57(s, 3H, CH3), 1.44(s, 3H, CH3), 1.39(s, 2×CH3) | 176.87, 155.10, 150.08, 147.91, 123.87, 116.58, 115.49, 114.50, 97.83, 61.54, 58.45, 56.06, 31.20, 24.97 |
| 16b | 7.67(d, J=8.5 Hz, 2H, ArH), 7.54(d, J=8.3 Hz, 2H, ArH), 6.51(s, 1H, NH), 6.16(s, 1H, CH), 2.57(s, 3H, CH3), 1.39(d, J=6.4 Hz, 6H, 2×CH3) | 176.80, 154.84, 132.51, 132.30, 131.95, 124.15, 95.43, 63.37, 58.44, 29.10, 24.98, 24.83 |
| 17b | 7.73(d, J=8.5 Hz, 2H, ArH), 7.38(d, J=8.5 Hz, 2H, ArH), 6.53—6.43(m, 1H, NH), 6.17(s, 1H, CH), 2.57(s, 3H, CH3), 1.39(d, J=4.1 Hz, 6H, 2×CH3) | 176.79, 154.84, 135.85, 132.24, 131.37, 128.95, 95.53, 63.29, 58.43, 29.10, 24.89 |
| 18b | 7.67(d, J=7.1 Hz, 2H, ArH), 7.54(d, J=5.5 Hz, 2H, ArH), 6.63(d, J=19.0 Hz, 1H, NH), 6.16(s, 1H, CH), 2.58(s, 3H, CH3), 1.40(d, J=2.8 Hz, 6H, 2×CH3) | 176.83, 154.86, 132.49, 132.29, 131.94, 124.14, 95.45, 63.37, 58.43, 29.11, 24.96, 24.80 |
| 19b | 8.26(d, J=8.8 Hz, 2H, ArH), 8.01(d, J=8.8 Hz, 2H, ArH), 6.51(s, 1H, NH), 6.30(s, 1H, CH), 2.59(s, 3H, CH3), 1.42(d, J=7.1 Hz, 6H, 2×CH3) | 176.73, 154.57, 148.50, 139.71, 131.95,131.36, 128.87, 123.79, 94.87, 62.98, 58.62, 29.33, 24.98, 24.85 |
| 20b | 8.74(s, 1H, ArH), 8.30(d, J=8.3 Hz, 1H, ArH), 8.17(d, J=7.8 Hz, 1H, ArH), 7.65(t, J=8.0 Hz, 1H, ArH), 6.85(s, 1H, NH), 6.31(s,1H, CH), 2.60(s, 3H, CH3), 1.44(s, 6H, 2×CH3) | 176.82, 154.81, 148.20, 136.88, 134.72, 129.87, 125.89, 124.58, 95.18, 62.94, 58.71, 29.22, 25.08, 24.86 |
Table 3 1H NMR and 13C NMR data for compounds 1b—20b
| Compd. | 1H NMR(300 MHz, CDCl3), δ | 13C NMR(75 MHz, CDCl3), δ |
|---|---|---|
| 1b | 7.74(dd, J=7.7, 1.5 Hz, 2H, ArH), 7.45—7.33(m, 3H, ArH), 6.80(s, 1H, NH), 6.46(s, 1H ,CH), 1.49(s, 3H ,CH3), 1.43(s, 3H, CH3) | 176.12, 154.95, 131.95, 130.55, 129.97, 128.60, 89.90, 66.11, 58.56, 25.19, 24.88 |
| 2b | 7.62(d, J=8.2 Hz, 2H, ArH), 7.17(d, J=8.1 Hz, 2H, ArH), 6.94(s, 1H , NH), 6.42(s, 1H, CH), 2.34(s, 3H, ArCH3), 1.48(s, 3H , CH3), 1.42(s, 3H, CH3) | 176.15, 155.01, 140.06, 130.45, 129.24, 128.87, 90.18, 65.96, 58.48, 25.11, 24.79, 21.20 |
| 3b | 7.70(d, J=8.9 Hz, 2H, ArH), 6.88(d, J=8.9 Hz, 2H , ArH), 6.60(s, 1H, NH), 6.42(s, 1H, CH), 3.81(s, 3H, OCH3), 1.49(s, 3H, CH3), 1.43(s, 3H, CH3) | 176.07, 160.67, 154.90, 132.30, 123.62, 113.84, 90.55, 65.86, 58.47, 55.26, 25.20, 24.89 |
| 4b | 7.07(s, 2H, ArH), 6.50(s, 1H, NH), 6.41(s, 1H, CH), 3.86(d, J=7.2 Hz, 9H, 3OCH3), 1.45(s, 3H, CH3) | 176.14, 154.72, 152.89, 139.31, 126.70, 108.54, 90.31, 66.42, 60.84, 58.43, 56.35, 25.24, 24.94 |
| 5b | 8.06(s, 1H, ArH), 7.04(s, 1H, NH), 6.76(s, 1H, NH), 6.74(s, 1H, CH), 3.91(s, 3H, OCH3), 3.88(s, 3H, OCH3), 1.47(s, 3H, CH3), 1.39(s, 3H, CH3) | 175.98, 154.58, 150.33, 147.37, 122.62,116.50, 115.21, 114.83, 90.34, 63.98, 58.43, 56.09, 56.08, 25.16, 24.84 |
| 6b | 7.79(d, J=3.7 Hz, 2H, ArH), 7.07(d, J=17.3 Hz, 2H ,ArH), 6.45(s, 1H, NH), 6.31(s, 1H, CH), 1.49(s, 3H, CH3), 1.42(s, 3H, CH3) | 176.07, 154.87, 131.95, 130.56, 129.95, 128.58, 89.89, 66.12, 58.53, 25.20, 24.90 |
| 7b | 7.72(d, J=8.4 Hz, 2H, ArH), 7.36(d, J=8.5 Hz, 2H, ArH), 6.52(s, 1H, NH), 6.41(s, 1H, CH), 1.46(d, J=22.2 Hz, 6H, 2CH3) | 175.93, 154.58, 136.28, 132.13, 130.28, 128.83, 89.45, 65.52, 58.61, 25.20, 24.92 |
| 8b | 7.65(d, J=8.5 Hz, 2H, ArH), 7.52(d, J=8.5 Hz, 2H, ArH), 6.68(s, 1H, NH), 6.39(s, 1H, CH), 1.49(s, 3H, CH3), 1.43(s, 3H, CH3) | 175.96, 154.69, 132.34, 131.81, 130.83, 124.62, 89.33, 65.60, 58.63, 25.17, 24.89 |
| 9b | 8.25(d, J=8.9 Hz, 2H, ArH), 7.98(d, J=8.8 Hz, 2H, ArH), 6.51(s, 1H, NH), 5.94(s, 1H, CH), 1.51(s, 3H, CH3), 1.45(s, 3H, CH3) | 175.70, 153.89, 148.63, 138.55, 131.81, 123.60 88.24, 65.14, 58.77, 25.24, 25.02 |
| 10b | 8.75(d, J=1.5 Hz, 1H , ArH), 8.30(d, J=9.6 Hz, 1H, ArH), 8.14(d, J=7.9 Hz, 1H, ArH), 7.62(t, J=8.1 Hz, 1H, ArH), 6.69(s, 1H, NH), 6.50(s, 1H, CH), 1.52(s, 3H, CH3), 1.47(s, 3H, CH3) | 175.96, 154.36, 148.03, 136.72, 133.66, 129.60, 126.00, 124.89, 88.53, 65.19, 58.87, 25.12, 24.92 |
| 11b | 8.55(d, J=7.5 Hz, 1H, ArH), 7.99(d, J=8.6 Hz, 1H, ArH), 7.89(dd, J=14.0, 8.1 Hz, 2H, ArH), 7.53(dd, J=22.9, 15.0, 7.4 Hz, 3H, ArH), 7.22(s, 1H, NH), 6.17(s, 1H,CH), 1.42(s, 3H, CH3), 1.27(s, 3H, CH3) | 175.84, 154.48, 133.87, 131.42, 130.59, 129.53, 128.57, 127.57, 126.50, 125.94, 124.43, 121.60, 89.73, 60.01, 58.47, 25.20, 24.79 |
| 12b | 7.77(dd, J=7.3, 2.1 Hz, 2H, ArH), 7.45—7.34(m, 3H, ArH), 6.69(s, 1H, NH), 6.20(s, 1H, CH), 2.59(s, 3H, CH3), 1.39(d, J=3.5 Hz, 6H, 2×CH3) | 176.99, 155.15, 132.96, 130.74, 129.54, 128.68, 95.75, 63.97, 58.35, 29.12, 24.93, 24.72 |
| 13b | 7.65(d, J=8.2 Hz, 2H, ArH), 7.20(d, J=8.0 Hz, 2H, ArH), 6.51(s, 1H, NH), 6.17(s, 1H, CH), 2.59(s, 3H, CH3), 2.37(ArCH3), 1.38(d, J=6.2 Hz, 6H, 2×CH3) | 176.84, 139.39, 130.54, 129.96, 129.16, 96.02, 63.77, 58.31, 29.11, 24.98, 24.80 |
| 14b | 7.71(d, J=8.8 Hz, 2H, ArH), 6.91(d, J=8.9 Hz, 2H, ArH), 6.48(s, 1H, NH), 6.15(s, 1H, CH), 3.82(s, 3H, OCH3), 2.58(s,3H, CH3), 1.38(d, J=4.5 Hz, 6H, 2×CH3) | 176.87, 160.38, 155.06, 132.31, 124.80, 113.98, 96.33, 63.55, 58.32, 55.29, 29.04, 24.90 |
| 15b | 7.91(s, 1H, ArH), 7.06(s, 1H, ArH), 6.76(s, 1H, NH), 6.65(s, 1H, CH), 3.88(s, 3H, OCH3), 3.87(s, 3H, OCH3), 2.57(s, 3H, CH3), 1.44(s, 3H, CH3), 1.39(s, 2×CH3) | 176.87, 155.10, 150.08, 147.91, 123.87, 116.58, 115.49, 114.50, 97.83, 61.54, 58.45, 56.06, 31.20, 24.97 |
| 16b | 7.67(d, J=8.5 Hz, 2H, ArH), 7.54(d, J=8.3 Hz, 2H, ArH), 6.51(s, 1H, NH), 6.16(s, 1H, CH), 2.57(s, 3H, CH3), 1.39(d, J=6.4 Hz, 6H, 2×CH3) | 176.80, 154.84, 132.51, 132.30, 131.95, 124.15, 95.43, 63.37, 58.44, 29.10, 24.98, 24.83 |
| 17b | 7.73(d, J=8.5 Hz, 2H, ArH), 7.38(d, J=8.5 Hz, 2H, ArH), 6.53—6.43(m, 1H, NH), 6.17(s, 1H, CH), 2.57(s, 3H, CH3), 1.39(d, J=4.1 Hz, 6H, 2×CH3) | 176.79, 154.84, 135.85, 132.24, 131.37, 128.95, 95.53, 63.29, 58.43, 29.10, 24.89 |
| 18b | 7.67(d, J=7.1 Hz, 2H, ArH), 7.54(d, J=5.5 Hz, 2H, ArH), 6.63(d, J=19.0 Hz, 1H, NH), 6.16(s, 1H, CH), 2.58(s, 3H, CH3), 1.40(d, J=2.8 Hz, 6H, 2×CH3) | 176.83, 154.86, 132.49, 132.29, 131.94, 124.14, 95.45, 63.37, 58.43, 29.11, 24.96, 24.80 |
| 19b | 8.26(d, J=8.8 Hz, 2H, ArH), 8.01(d, J=8.8 Hz, 2H, ArH), 6.51(s, 1H, NH), 6.30(s, 1H, CH), 2.59(s, 3H, CH3), 1.42(d, J=7.1 Hz, 6H, 2×CH3) | 176.73, 154.57, 148.50, 139.71, 131.95,131.36, 128.87, 123.79, 94.87, 62.98, 58.62, 29.33, 24.98, 24.85 |
| 20b | 8.74(s, 1H, ArH), 8.30(d, J=8.3 Hz, 1H, ArH), 8.17(d, J=7.8 Hz, 1H, ArH), 7.65(t, J=8.0 Hz, 1H, ArH), 6.85(s, 1H, NH), 6.31(s,1H, CH), 2.60(s, 3H, CH3), 1.44(s, 6H, 2×CH3) | 176.82, 154.81, 148.20, 136.88, 134.72, 129.87, 125.89, 124.58, 95.18, 62.94, 58.71, 29.22, 25.08, 24.86 |
| Entry | Substrate | t/℃ | Catalyst | Molar fraction of cat.(%) | Solvent | Time/h | Product | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 25 | K3PO4 | 20 | CH3CN | 24 | 1b | 91 |
| 2 | 1a | 25 | K2CO3 | 20 | CH3CN | 24 | 1b | 82 |
| 3 | 1a | 25 | Na2CO3 | 20 | CH3CN | 13 | 1b | 94 |
| 4 | 1a | 25 | NaOH | 20 | CH3CN | 13 | 1b | 49 |
| 5 | 1a | 25 | KOH | 20 | CH3CN | 24 | 1b | 79 |
| 6 | 1a | 25 | CH3ONa | 20 | CH3CN | 24 | 1b | 74 |
| 7 | 1a | 25 | Triethylamine | 20 | CH3CN | 24 | NRc | |
| 8 | 1a | 25 | 1,2-Diaminoethane | 20 | CH3CN | 24 | NRc | |
| 9 | 1a | 25 | Pyridine | 20 | CH3CN | 12 | NRc | |
| 10 | 1a | 25 | Piperidine | 20 | CH3CN | 24 | NRc | |
| 11 | 1a | 25 | Na2CO3 | 50 | CH3CN | 6 | 1b | 80 |
| 12 | 1a | 25 | Na2CO3 | 10 | CH3CN | 24 | 1b | 61 |
| 13 | 1a | 25 | No | 0 | CH3CN | 24 | 1b | Trace |
| 14 | 1a | 25 | Na2CO3 | 20 | CH2Cl2 | 24 | NRc | |
| 15 | 1a | 25 | Na2CO3 | 20 | CH3OH | 24 | NRc | |
| 16 | 1a | 25 | Na2CO3 | 20 | CH3COCH3 | 8 | 1b | 66 |
| 17 | 1a | 25 | Na2CO3 | 20 | DMF | 24 | 1b | 85 |
| 18 | 1a | 25 | Na2CO3 | 20 | C6H5CH3 | 24 | NRc | |
| 19 | 1a | 25 | Na2CO3 | 20 | THF | 24 | NRc | |
| 20 | 12a | 25 | Na2CO3 | 20 | CH3CN | 24 | NRc | |
| 21 | 12a | 40 | Na2CO3 | 20 | CH3CN | 24 | 12b | 26 |
| 22 | 12a | 60 | Na2CO3 | 20 | CH3CN | 12 | 12b | 42 |
| 23 | 12a | Reflux | Na2CO3 | 20 | CH3CN | 8 | 12b | 68 |
| 24 | 12a | Reflux | KOH | 20 | CH3CN | 7 | 12b | 93 |
| 25 | 12a | Reflux | K3PO3 | 20 | CH3CN | 12 | 12b | 61 |
| 26 | 12a | Reflux | K2CO3 | 20 | CH3CN | 12 | 12b | 69 |
| 27 | 12a | Reflux | NaOH | 20 | CH3CN | 5 | 12b | 85 |
| 28 | 12a | Reflux | CH3ONa | 20 | CH3CN | 1 | 12b | 73 |
| 29 | 12a | Reflux | Triethylamine | 20 | CH3CN | 24 | NRc | |
| 30 | 12a | Reflux | 1,2-Diaminoethane | 20 | CH3CN | 24 | NRc | |
| 31 | 12a | Reflux | Pyridine | 20 | CH3CN | 24 | NRc | |
| 32 | 12a | Reflux | KOH | 50 | CH3CN | 7 | 12b | 90 |
| 33 | 12a | Reflux | KOH | 10 | CH3CN | 10 | 12b | 90 |
| 34 | 12a | Reflux | KOH | 5 | CH3CN | 15 | 12b | 78 |
| 35 | 12a | Reflux | None | 0 | CH3CN | 24 | 12b | 40 |
Table 4 Effects of catalyst and temperature as well as solvent to the aminobromination of β-nitrostyrenea
| Entry | Substrate | t/℃ | Catalyst | Molar fraction of cat.(%) | Solvent | Time/h | Product | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 25 | K3PO4 | 20 | CH3CN | 24 | 1b | 91 |
| 2 | 1a | 25 | K2CO3 | 20 | CH3CN | 24 | 1b | 82 |
| 3 | 1a | 25 | Na2CO3 | 20 | CH3CN | 13 | 1b | 94 |
| 4 | 1a | 25 | NaOH | 20 | CH3CN | 13 | 1b | 49 |
| 5 | 1a | 25 | KOH | 20 | CH3CN | 24 | 1b | 79 |
| 6 | 1a | 25 | CH3ONa | 20 | CH3CN | 24 | 1b | 74 |
| 7 | 1a | 25 | Triethylamine | 20 | CH3CN | 24 | NRc | |
| 8 | 1a | 25 | 1,2-Diaminoethane | 20 | CH3CN | 24 | NRc | |
| 9 | 1a | 25 | Pyridine | 20 | CH3CN | 12 | NRc | |
| 10 | 1a | 25 | Piperidine | 20 | CH3CN | 24 | NRc | |
| 11 | 1a | 25 | Na2CO3 | 50 | CH3CN | 6 | 1b | 80 |
| 12 | 1a | 25 | Na2CO3 | 10 | CH3CN | 24 | 1b | 61 |
| 13 | 1a | 25 | No | 0 | CH3CN | 24 | 1b | Trace |
| 14 | 1a | 25 | Na2CO3 | 20 | CH2Cl2 | 24 | NRc | |
| 15 | 1a | 25 | Na2CO3 | 20 | CH3OH | 24 | NRc | |
| 16 | 1a | 25 | Na2CO3 | 20 | CH3COCH3 | 8 | 1b | 66 |
| 17 | 1a | 25 | Na2CO3 | 20 | DMF | 24 | 1b | 85 |
| 18 | 1a | 25 | Na2CO3 | 20 | C6H5CH3 | 24 | NRc | |
| 19 | 1a | 25 | Na2CO3 | 20 | THF | 24 | NRc | |
| 20 | 12a | 25 | Na2CO3 | 20 | CH3CN | 24 | NRc | |
| 21 | 12a | 40 | Na2CO3 | 20 | CH3CN | 24 | 12b | 26 |
| 22 | 12a | 60 | Na2CO3 | 20 | CH3CN | 12 | 12b | 42 |
| 23 | 12a | Reflux | Na2CO3 | 20 | CH3CN | 8 | 12b | 68 |
| 24 | 12a | Reflux | KOH | 20 | CH3CN | 7 | 12b | 93 |
| 25 | 12a | Reflux | K3PO3 | 20 | CH3CN | 12 | 12b | 61 |
| 26 | 12a | Reflux | K2CO3 | 20 | CH3CN | 12 | 12b | 69 |
| 27 | 12a | Reflux | NaOH | 20 | CH3CN | 5 | 12b | 85 |
| 28 | 12a | Reflux | CH3ONa | 20 | CH3CN | 1 | 12b | 73 |
| 29 | 12a | Reflux | Triethylamine | 20 | CH3CN | 24 | NRc | |
| 30 | 12a | Reflux | 1,2-Diaminoethane | 20 | CH3CN | 24 | NRc | |
| 31 | 12a | Reflux | Pyridine | 20 | CH3CN | 24 | NRc | |
| 32 | 12a | Reflux | KOH | 50 | CH3CN | 7 | 12b | 90 |
| 33 | 12a | Reflux | KOH | 10 | CH3CN | 10 | 12b | 90 |
| 34 | 12a | Reflux | KOH | 5 | CH3CN | 15 | 12b | 78 |
| 35 | 12a | Reflux | None | 0 | CH3CN | 24 | 12b | 40 |
| Entry | Substrate | Product | Time/h | Yieldb(%) | Entry | Substrate | Product | Time/h | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 1b | 13 | 94 | 11 | 11a | 11b | 24 | 90 |
| 2 | 2a | 2b | 24 | 89 | 12 | 12a | 12b | 10 | 90 |
| 3 | 3a | 3b | 24 | 85 | 13 | 13a | 13b | 15 | 78 |
| 4 | 4a | 4b | 24 | 45 | 14 | 14a | 14b | 24 | 76 |
| 5 | 5a | 5b | 24 | 65 | 15 | 15a | 15b | 24 | 84 |
| 6 | 6a | 6b | 8 | 95 | 16 | 16a | 16b | 5 | 95 |
| 7 | 7a | 7b | 8 | 95 | 17 | 17a | 17b | 8 | 94 |
| 8 | 8a | 8b | 8 | 94 | 18 | 18a | 18b | 8 | 94 |
| 9 | 9a | 9b | 6 | 97 | 19 | 19a | 19b | 6 | 95 |
| 10 | 10a | 10b | 6 | 96 | 20 | 20a | 20b | 7 | 93 |
Table 5 Aminobromination of β-nitrostyren derivatives with DBDMH catalyzed by basea
| Entry | Substrate | Product | Time/h | Yieldb(%) | Entry | Substrate | Product | Time/h | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 1b | 13 | 94 | 11 | 11a | 11b | 24 | 90 |
| 2 | 2a | 2b | 24 | 89 | 12 | 12a | 12b | 10 | 90 |
| 3 | 3a | 3b | 24 | 85 | 13 | 13a | 13b | 15 | 78 |
| 4 | 4a | 4b | 24 | 45 | 14 | 14a | 14b | 24 | 76 |
| 5 | 5a | 5b | 24 | 65 | 15 | 15a | 15b | 24 | 84 |
| 6 | 6a | 6b | 8 | 95 | 16 | 16a | 16b | 5 | 95 |
| 7 | 7a | 7b | 8 | 95 | 17 | 17a | 17b | 8 | 94 |
| 8 | 8a | 8b | 8 | 94 | 18 | 18a | 18b | 8 | 94 |
| 9 | 9a | 9b | 6 | 97 | 19 | 19a | 19b | 6 | 95 |
| 10 | 10a | 10b | 6 | 96 | 20 | 20a | 20b | 7 | 93 |
| [1] | Kemp J.E. G., Fleming M. I., Comprehensive Organic Synthesis, Pergamon, Oxford, 1991, 471—513 |
| [2] | Chen Z. G., Wang D., Li Y. N., Wang Y. J., Hu J. L., Xia W., Acta Chim. Sinica, 2012, 70(21), 2236—2245 |
| (陈战国, 王丹, 李亚男, 王英杰, 胡均利, 夏伟.化学学报, 2012,70(21), 2236—2245) | |
| [3] | Wen H., Wang Y. J., Liu D. E., Liu Y. L., Ge M., Chen Z. G., Chin. J. Org. Chem., 2014, 34(5), 916—925 |
| (闻化, 王英杰, 刘德娥, 刘亚丽, 葛淼, 陈战国.有机化学, 2014,34(5), 916—925) | |
| [4] | Chen Z. G., Wang Y. J., Liu D. E., Liu Y. L., Li Y. N., Wang D., Ge M., Chem. J. Chinese Universities, 2014, 35(7), 1458—1464 |
| (陈战国, 王英杰, 刘德娥, 刘亚丽, 李亚男, 王丹, 葛淼.高等学校化学学报, 2014,35(7), 1458—1464) | |
| [5] | Chen Z. G., Liu D. E., Li W. L., Liu Y. L., Chem. J. Chinese Universities, 2014, 35(11), 2360—2365 |
| (陈战国, 刘德娥, 李文丽, 刘亚丽.高等学校化学学报, 2014,35(11), 2360—2365) | |
| [6] | Chen Z. G., Wei J. F., Wang M. Z., Zhou L. Y., Zhang C. J., Shi X. Y., Adv. Synth. Catal., 2009, 351, 2358—2368 |
| [7] | Liu J. Y., Wang Y. I., Li G., Eur. J. Org. Chem., 2006, 14, 3112—3115 |
| [8] | Chen Z.G., Wang Y., Wei J. F., Zhao P. F., Shi X. Y., J. Org. Chem., 2010, 75, 2085—2088 |
| [9] | Zhi S. J., Sun H., Zhang G. Q., Li G., Pan Y., Org. Biomol. Chem., 2010, 8, 628—631 |
| [10] | Kansal V. K., Bhaduri A. P., Indian J. Chem., Sect. B, 1981, 20(10), 885—890 |
| [11] | Enders D., Wiedemann J., Synthesis, 1996, 12, 1443—1450 |
| [12] | Zhi S. J., Han J., Lin C., An G. H., Pan Y., Li G., Synthesis, 2008, 10, 1570—1574 |
| [13] | Chen Z. G., Wei J. F., Li R. T., Shi X. Y., Zhao P. F., J. Org. Chem., 2009, 74, 1371—1373 |
| [14] | Chen Z. G., Li Y. N., Zhou J. M., Wang D., Ge M., Chem. Res. Chinese Universities, 2014, 30(2), 266—271 |
| [15] | Chen Z. G., Liu Y. L., Hu J. L., Liu D. E., Chem. Res. Chinese Universities, 2015, 31(1), 65—70 |
| [16] | Chen Z.G., Wang Y., Wei J. F., Zhao P. F., Shi X. Y., J. Org. Chem., 2010, 75, 2085—2088 |
| [17] | Handzlik J., Bojarski A. J., Satala G., Kubacka M., Sadek B., Ashoor A., Siwek A., Wiecek M., Kucwaj K., Filipek B., Eur. J. Med. Chem., 2014, 78, 324—339 |
| [18] | Gryder B. E., Akbashev M. J., Rood M. K., Raftery E. D., Meyers W. M., Dillard P., Khan S., Oyelere A. K.,ACS Chemical Biology, 2013, 8(11), 2550—2560 |
| [19] | Ning P. Y., Ding J. H., Shen P., Su X., Chinese Journal of Disinfection, 2011, 28(3), 270—271 |
| (宁培勇, 丁津华, 沈芃, 苏旭.中国消毒学杂志, 2011,28(3), 270—271) | |
| [20] | Shibatomi K., Zhang Y., Yamamoto H., Chem. Asian J., 2008, 3, 1581—1584 |
| [21] | Gairaud C. B., Lappin G. R., J. Org. Chem., 1953, 18, 1—3 |
| [22] | Michael A. B., Dieter S., Can. J. Chem., 1987, 65(4), 836—850 |
| [23] | Toshiyuki O., Sakae U., Bull. Chem. Soc. Jpn., 2003, 76(7), 1423—1431 |
| [24] | Dauzonne D., Royer R. S. C., Chem. Pharm. Bull., 1986, 34(4), 1628—1633 |
| [25] | Wang Y., Study on the Aminobromination of β-Nitrostyrene Derivatives with Acetamide and NBS, Shaanxi Normal University, Xi’an, 2010 |
| (王芸. β-硝基苯乙烯与乙酰胺/N-溴代丁二酰亚胺的氨溴加成反应研究, 西安: 陕西师范大学, 2010) | |
| [26] | Sandhar R. K., Sharma J. R., Manrao M. R.,J. Ind. Chem. Soc., 2006, 83(3), 263—265 |
| [27] | Ohwada T., Ohta T., Shudo K., Tetrahedron, 1987, 43(2), 297—305 |
| [28] | Ohe T., Uemura S., Bull.Chem. Soc. Jpn., 2003, 76(7), 1423—1431 |
| [29] | Nishimura T., Bull. Chem. Soc. Jpn., 1954, 27(9), 617—619 |
| [30] | Ohe T., Bull. Chem. Soc. Jpn., 2003, 76(7), 1423—1431 |
| [31] | Jakubec P., Tetrahedron, 2011, 22(11), 1147—1155 |
| [32] | Calheiros R., Milhazes N., Borges F., J. Mol. Struct., 2004, 692(1—3), 91—106 |
| [33] | Worrall D. E., J. Am. Chem. Soc., 1938, 60(12), 2841—2844 |
| [34] | Rokhum L., Bez G., Tetrahedron Letters, 2013, 54(40), 5500—5504 |
| [35] | Agafonov N. E., Sedishev I. P., Dudin A. V., IAN. Seriya Khimicheskaya, 1991, 2, 426—433 |
| [36] | Nguyen B., Katya C., Daniel E., Angew. Chem. Int. Ed., 2007, 46(40), 7655—7658 |
| [37] | Gross A., Schneiders N., Daniel K., Tetrahedron, 2008, 64(48), 10882—10889 |
| [1] | DU Manfei, HOU Dan, HUI Wenping, CHEN Zhanguo. Regiospecific Aminobromination of β-Nitrostyrene Derivatives with Acrylamide and N-Bromobutanimide† [J]. Chem. J. Chinese Universities, 2016, 37(5): 902. |
| [2] | CHEN Zhan-Guo, HU Jun-Li, XIA Wei, WANG Dan, LI Yan-Nan. Regio- and Stereoselective Aminobromination of Olefins with p-Toluenesulfonamide and 1,3-Dibromo-5,5-dimethyl Hydantoin Catalyzed by a New Catalyst Produced In-situ from Zinc Powder [J]. Chem. J. Chinese Universities, 2013, 34(5): 1151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||