

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (4): 20220616.doi: 10.7503/cjcu20220616
收稿日期:2022-09-15
出版日期:2023-04-10
发布日期:2022-11-03
通讯作者:
姚丽
E-mail:yaoli@dicp.ac.cn
基金资助:
XIA Wenwen1, YU Hongjing1, WANG Shiye1, YAO Li2( ), LI Xiangyuan3
), LI Xiangyuan3
Received:2022-09-15
Online:2023-04-10
Published:2022-11-03
Contact:
YAO Li
E-mail:yaoli@dicp.ac.cn
Supported by:摘要:
基于极小反应网络(MRN)方法, 在燃烧机理的化学分辨率(化学物种数)确定的条件下, 构建了极小反应网络的苯、 甲苯、 乙苯和丙苯通用复杂燃烧反应机理, 机理分别由22个物种和35个反应、 27个物种和42个反应、 32个物种和58个反应以及36个物种和68个反应组成. 建模方法是在极小网络C3机理基础上增加5个物种和14个反应构建苯燃烧机理, 增加7个物种和15个反应得到甲苯燃烧机理; 在苯燃烧机理基础上增加4个物种和8个反应构建乙苯燃烧机理, 增加3个物种和7个反应得到丙苯燃烧机理. 各个机理均采用Arrhenius方程的双参数形式(A, E)描述反应的速率常数. 通过点火延迟时间和层流火焰速度的动力学模拟与实验结果的对比, 验证各个燃烧反应机理的可靠性和实用性.
中图分类号:
TrendMD:
夏文文, 于洪晶, 王时野, 姚丽, 李象远. 用于燃烧反应机理构建的极小反应网络方法—芳香烃燃烧. 高等学校化学学报, 2023, 44(4): 20220616.
XIA Wenwen, YU Hongjing, WANG Shiye, YAO Li, LI Xiangyuan. Combustion Mechanism Construction Based on Minimized Reaction Network: Combustion of Aromatic Hydrocarbon. Chem. J. Chinese Universities, 2023, 44(4): 20220616.
| Label | Species | C6H6 | O2 | H2 | Label | Species | C6H6 | O2 | H2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | cyc⁃C6H5 | 1 | 0 | -1/2 | 11 | HCO | 1/6 | 1/2 | 0 |
| 2 | cyc⁃C6H5O | 1 | 1/2 | -1/2 | 12 | CO | 1/6 | 1/2 | -1/2 |
| 3 | C5H5 | 5/6 | 0 | 0 | 13 | CO2 | 1/6 | 1 | -1/2 |
| 4 | C4H3 | 4/6 | 0 | -1/2 | 14 | H2O2 | 0 | 1 | 1 |
| 5 | C3H3 | 1/2 | 0 | 0 | 15 | H2O | 0 | 1/2 | 1 |
| 6 | C2H2 | 1/3 | 0 | 0 | 16 | HO2 | 0 | 1 | 1/2 |
| 7 | C2H | 1/3 | 0 | -1/2 | 17 | OH | 0 | 1/2 | 1/2 |
| 8 | CH2CO | 1/3 | 1/2 | 0 | 18 | H | 0 | 0 | 1/2 |
| 9 | CH2O | 1/6 | 1/2 | 1/2 | 19 | O | 0 | 1/2 | 0 |
| 10 | CH2OH | 1/6 | 1/2 | 1 |
Table 1 Stoichiometric coefficient matrix of benzene combustion mechanism
| Label | Species | C6H6 | O2 | H2 | Label | Species | C6H6 | O2 | H2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | cyc⁃C6H5 | 1 | 0 | -1/2 | 11 | HCO | 1/6 | 1/2 | 0 |
| 2 | cyc⁃C6H5O | 1 | 1/2 | -1/2 | 12 | CO | 1/6 | 1/2 | -1/2 |
| 3 | C5H5 | 5/6 | 0 | 0 | 13 | CO2 | 1/6 | 1 | -1/2 |
| 4 | C4H3 | 4/6 | 0 | -1/2 | 14 | H2O2 | 0 | 1 | 1 |
| 5 | C3H3 | 1/2 | 0 | 0 | 15 | H2O | 0 | 1/2 | 1 |
| 6 | C2H2 | 1/3 | 0 | 0 | 16 | HO2 | 0 | 1 | 1/2 |
| 7 | C2H | 1/3 | 0 | -1/2 | 17 | OH | 0 | 1/2 | 1/2 |
| 8 | CH2CO | 1/3 | 1/2 | 0 | 18 | H | 0 | 0 | 1/2 |
| 9 | CH2O | 1/6 | 1/2 | 1/2 | 19 | O | 0 | 1/2 | 0 |
| 10 | CH2OH | 1/6 | 1/2 | 1 |
| Species | Reaction | Species | Reaction |
|---|---|---|---|
| C6H6 | C6H6+O2 | cyc⁃C6H5 | cyc⁃C6H5+HO2 |
| C6H6+O2 | cyc⁃C6H5+O2 | ||
| C6H6+O | cyc⁃C6H5 | ||
| C6H6+HO2 | cyc⁃C6H5O | cyc⁃C6H5O | |
| C6H6+OH | C5H5 | C5H5 | |
| C6H6+H | C4H3 | C4H3 | |
| C6H6 | |||
| C6H6 |
Table 2 New reactions in benzene combustion mechanism
| Species | Reaction | Species | Reaction |
|---|---|---|---|
| C6H6 | C6H6+O2 | cyc⁃C6H5 | cyc⁃C6H5+HO2 |
| C6H6+O2 | cyc⁃C6H5+O2 | ||
| C6H6+O | cyc⁃C6H5 | ||
| C6H6+HO2 | cyc⁃C6H5O | cyc⁃C6H5O | |
| C6H6+OH | C5H5 | C5H5 | |
| C6H6+H | C4H3 | C4H3 | |
| C6H6 | |||
| C6H6 |
| Species | Reaction | Species | Reaction |
|---|---|---|---|
| C6H5CH3 | C6H5CH3+O2 | C6H5CH2 | C6H5CH2 |
| C6H5CH3+O2 | C6H5CH2O | C6H5CH2O | |
| C6H5CH3+OH | cyc⁃C6H5O | cyc⁃C6H5O | |
| C6H5CH3+OH | cyc⁃C6H5 | cyc⁃C6H5+O2 | |
| C6H5CH3+H | cyc⁃C6H5+HO2 | ||
| C6H5CH3 | cyc⁃C6H5 | ||
| C6H5CH3 | C5H5 | C5H5 | |
| C4H3 | C4H3 |
Table 3 New reactions in toluene combustion mechanism
| Species | Reaction | Species | Reaction |
|---|---|---|---|
| C6H5CH3 | C6H5CH3+O2 | C6H5CH2 | C6H5CH2 |
| C6H5CH3+O2 | C6H5CH2O | C6H5CH2O | |
| C6H5CH3+OH | cyc⁃C6H5O | cyc⁃C6H5O | |
| C6H5CH3+OH | cyc⁃C6H5 | cyc⁃C6H5+O2 | |
| C6H5CH3+H | cyc⁃C6H5+HO2 | ||
| C6H5CH3 | cyc⁃C6H5 | ||
| C6H5CH3 | C5H5 | C5H5 | |
| C4H3 | C4H3 |
| Species | Reaction | Species | Reaction |
|---|---|---|---|
| C6H5C2H5 | C6H5C2H5+O2 | C6H5C2H4 | C6H5C2H4 |
| C6H5C2H5+H | C6H5C2H4+O2 | ||
| C6H5C2H5 | C6H5C2H3 | C6H5C2H3 | |
| C6H5C2H5 | C6H5CH2 | C6H5CH2 |
Table 4 New reactions in ethylbenzene combustion mechanism
| Species | Reaction | Species | Reaction |
|---|---|---|---|
| C6H5C2H5 | C6H5C2H5+O2 | C6H5C2H4 | C6H5C2H4 |
| C6H5C2H5+H | C6H5C2H4+O2 | ||
| C6H5C2H5 | C6H5C2H3 | C6H5C2H3 | |
| C6H5C2H5 | C6H5CH2 | C6H5CH2 |
| Species | Reaction |
|---|---|
| C6H5C3H7 | C6H5C3H7+O2 |
| C6H5C3H7+HO2 | |
| C6H5C3H7+H | |
| C6H5C3H7 | |
| C6H5C3H7 | |
| C6H5C3H6 | C6H5C3H6 |
| C6H5CH2 | C6H5CH2 |
Table 5 New reactions in n-propylbenzene combustion mechanism
| Species | Reaction |
|---|---|
| C6H5C3H7 | C6H5C3H7+O2 |
| C6H5C3H7+HO2 | |
| C6H5C3H7+H | |
| C6H5C3H7 | |
| C6H5C3H7 | |
| C6H5C3H6 | C6H5C3H6 |
| C6H5CH2 | C6H5CH2 |
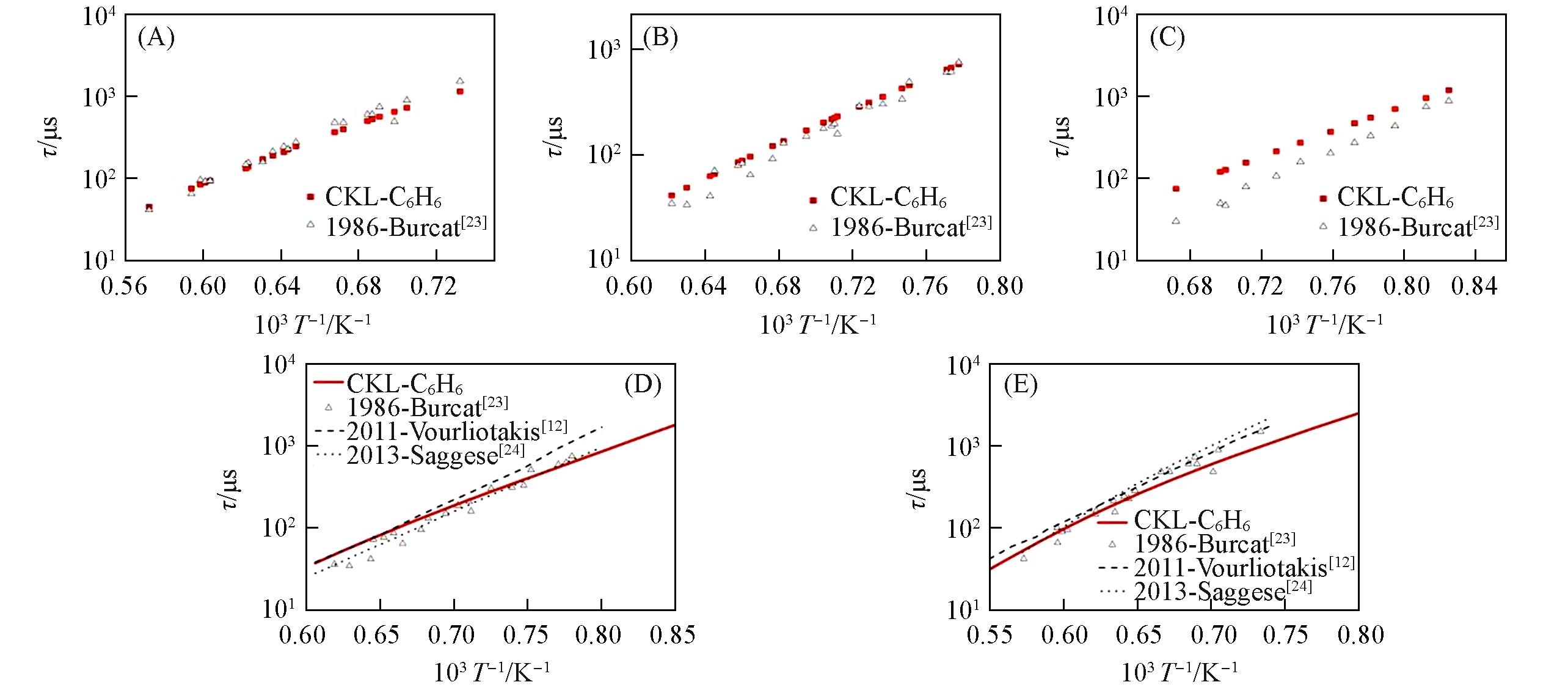
Fig.1 Ignition delay time(τ) for benzene/O2/Ar mixtures predicted by different mechanisms compared with experimental data(A) p=0.208—0.306 MPa, ɸ=2.0; (B) p=0.210—0.305 MPa, ɸ=1.0; (C) p=0.193—0.277 MPa, ɸ=0.5; (D) p=0.247 MPa, ɸ=1.0; (E) p=0.247 MPa, ɸ=2.0.
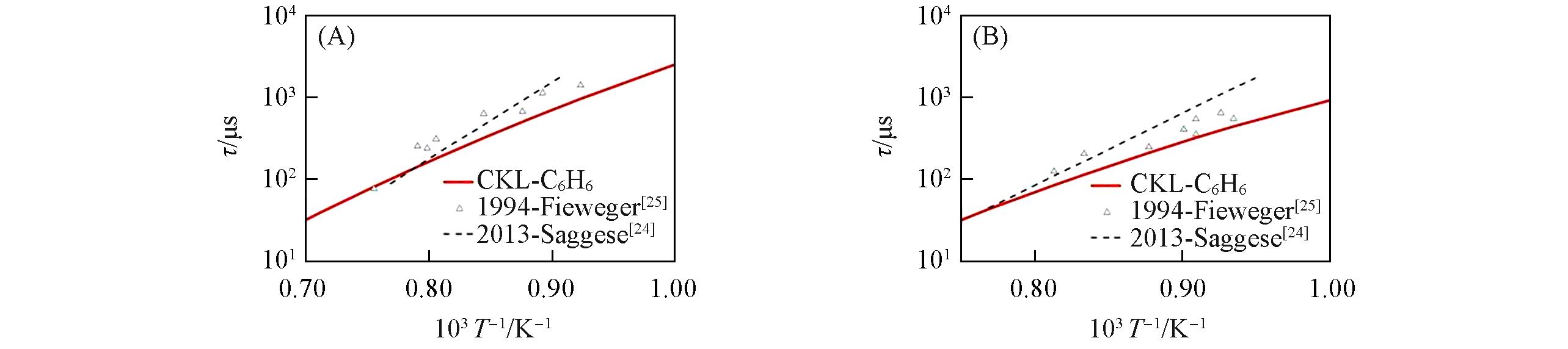
Fig.2 Ignition delay time for benzene/O2/N2 mixtures predicted by different mechanisms compared with experimental data(A) p=1.317 MPa, ɸ=1.0; (B) p=3.952 MPa, ɸ=1.0.
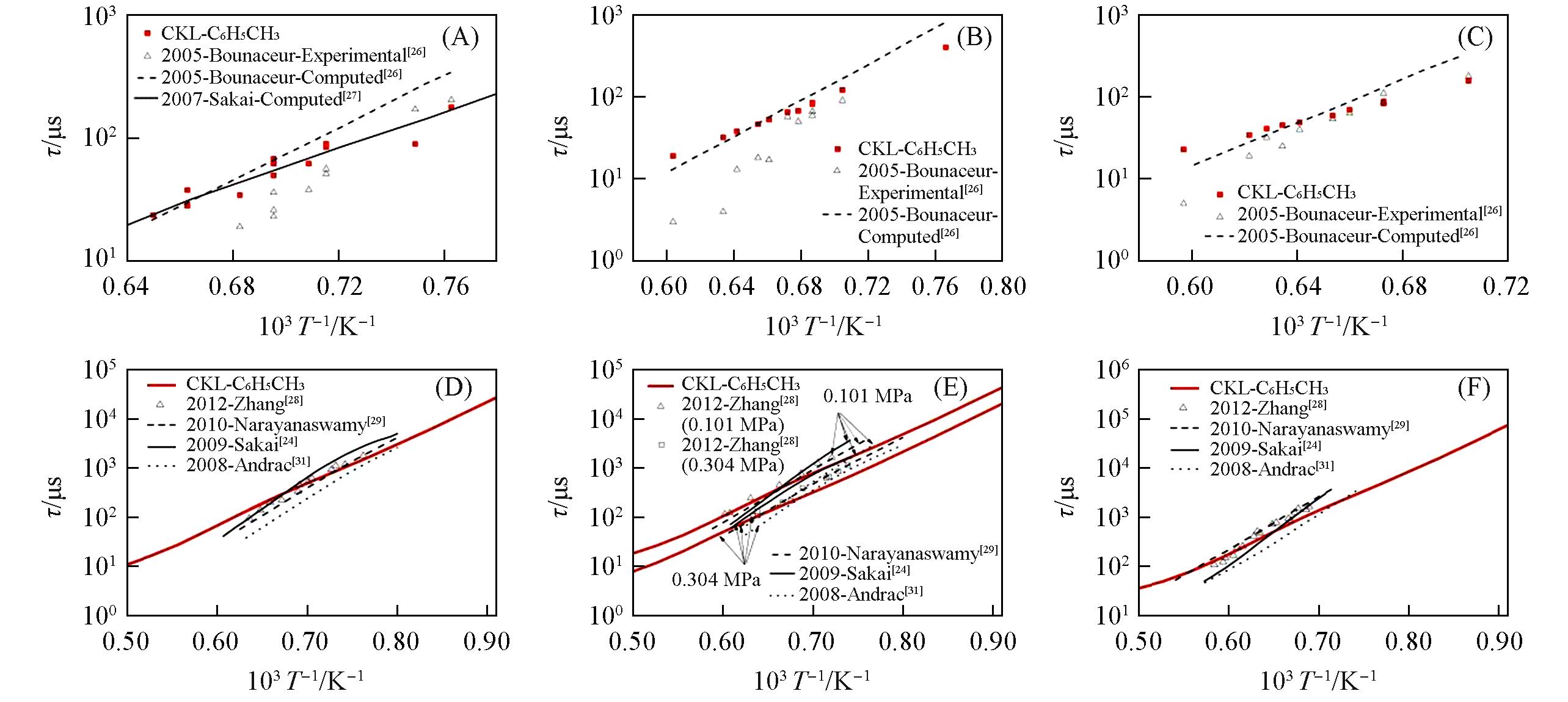
Fig.3 Ignition delay time for toluene/O2/Ar mixtures predicted by different mechanisms compared with experimental data(A) p=0.817—0.958 MPa, ɸ=0.5; (B) p=0.836—0.955 MPa, ɸ=1.0; (C) p=0.833—0.919 MPa, ɸ=1.5; (D) p=0.101 MPa, ɸ=0.5(18%O2); (E) p=0.101 MPa, p=0.304 MPa, ɸ=1.0(9.0%O2); (F) p=0.101 MPa, ɸ=2.0(4.5%O2).
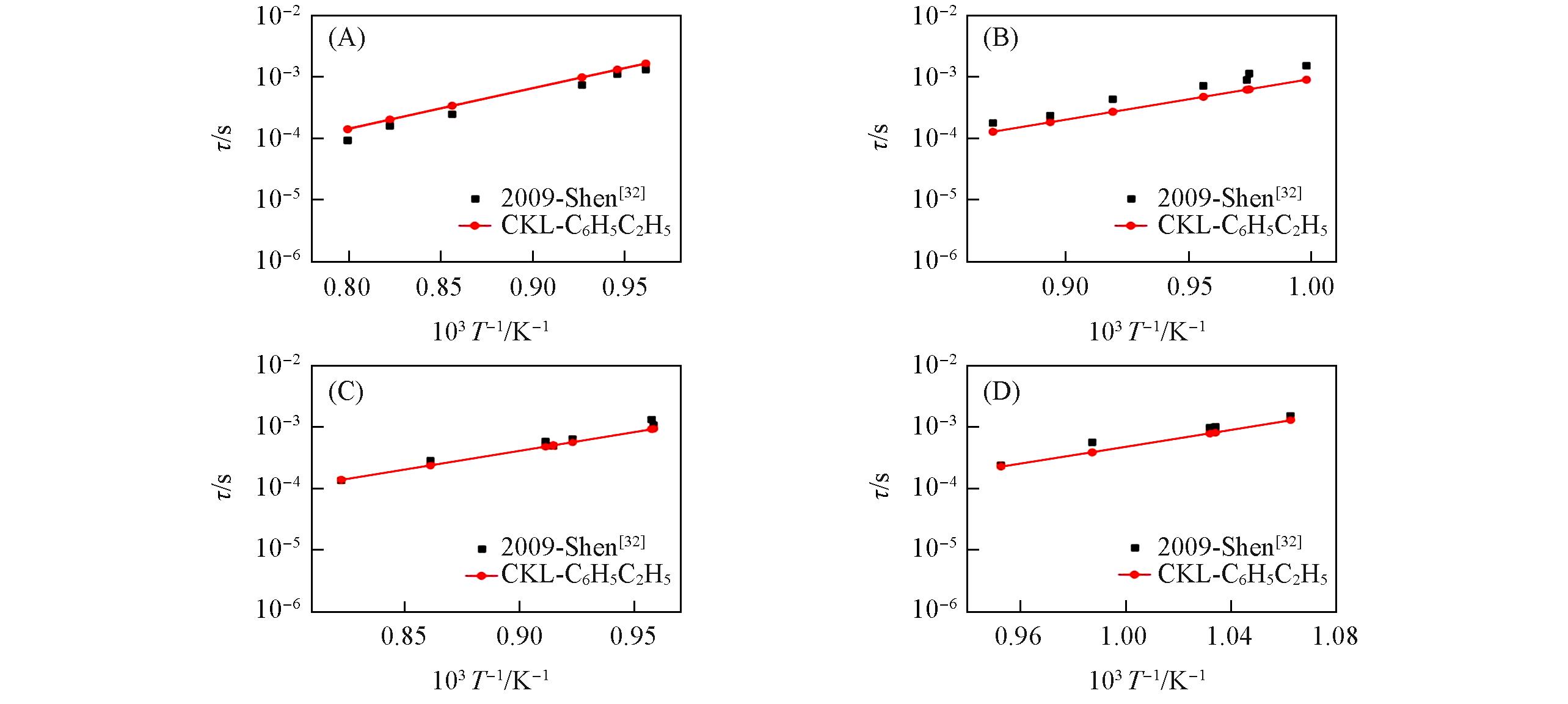
Fig.4 Ignition delay time for ethylbenzene/O2/N2 mixtures predicted by different mechanisms compared with experimental data(A) p=1.013 MPa, ɸ=0.5; (B) p=4.053 MPa, ɸ=0.5; (C) p=1.013 MPa, ɸ=1.0; (D) p=4.053 MPa, ɸ=1.0.
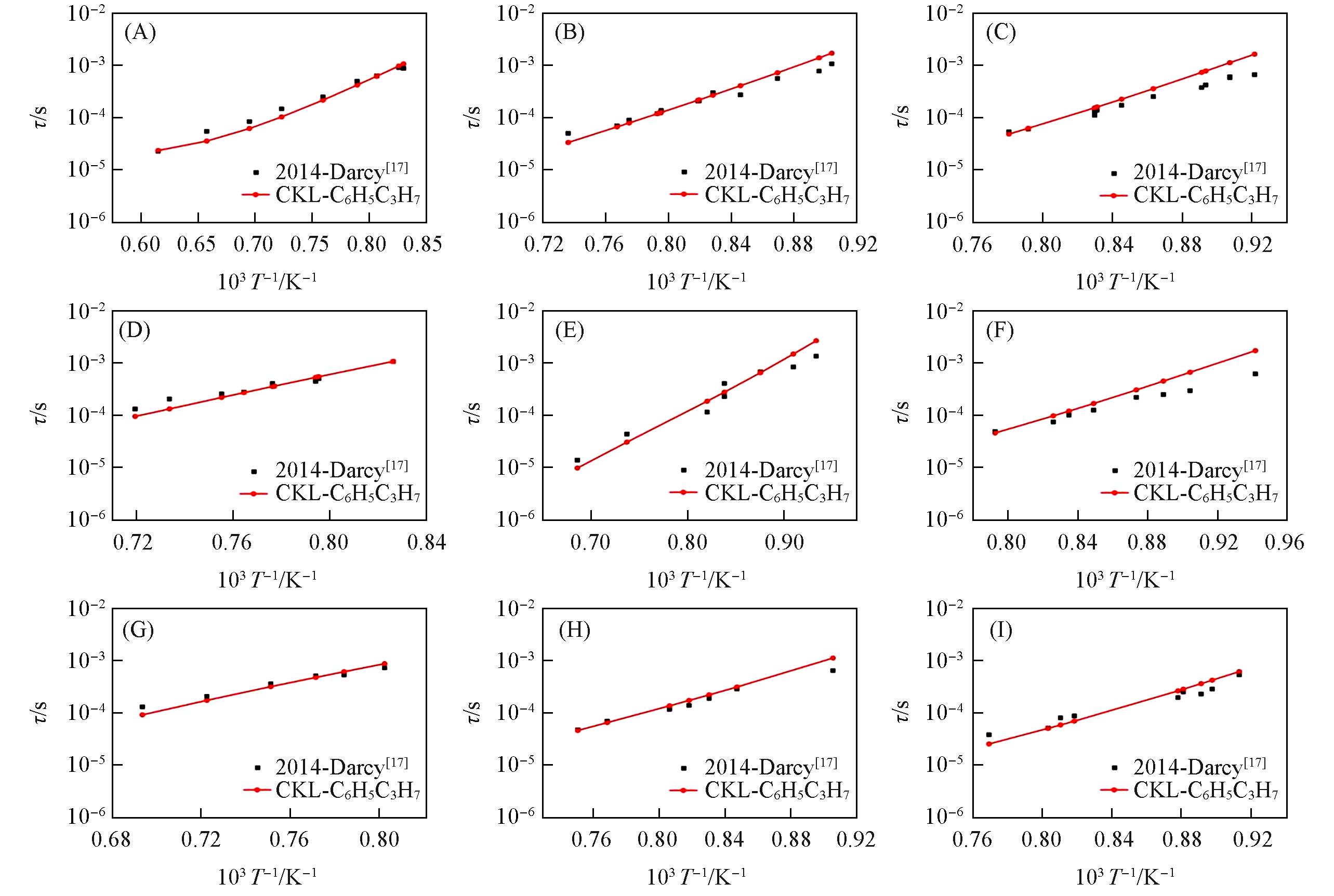
Fig.5 Ignition delay time for n⁃propylbenzene/O2/N2 mixtures predicted by different mechanisms compared with experimental data(A) p=0.101 MPa, ɸ=0.48; (B) p=1.013 MPa, ɸ=0.48; (C) p=3.040 MPa, ɸ=0.48; (D) p=0.101 MPa, ɸ=0.96; (E) p=1.013 MPa, ɸ=0.96; (F) p=3.040 MPa, ɸ=0.96; (G) p=0.101 MPa, ɸ=1.92; (H) p=1.013 MPa, ɸ=1.92; (I) p=3.040 MPa, ɸ=1.92.
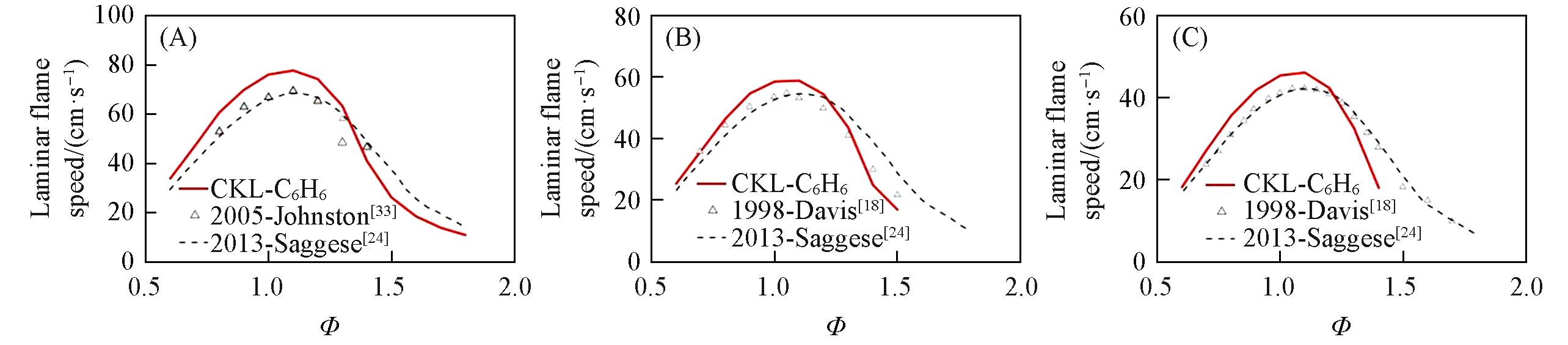
Fig.6 Laminar flame speed for benzene/air mixtures predicted by different mechanisms compared with experimental data(A) p=0.304 MPa, T=450 K; (B) p=0.101 MPa, T=353 K; (C) p=0.101 MPa, T=298 K.
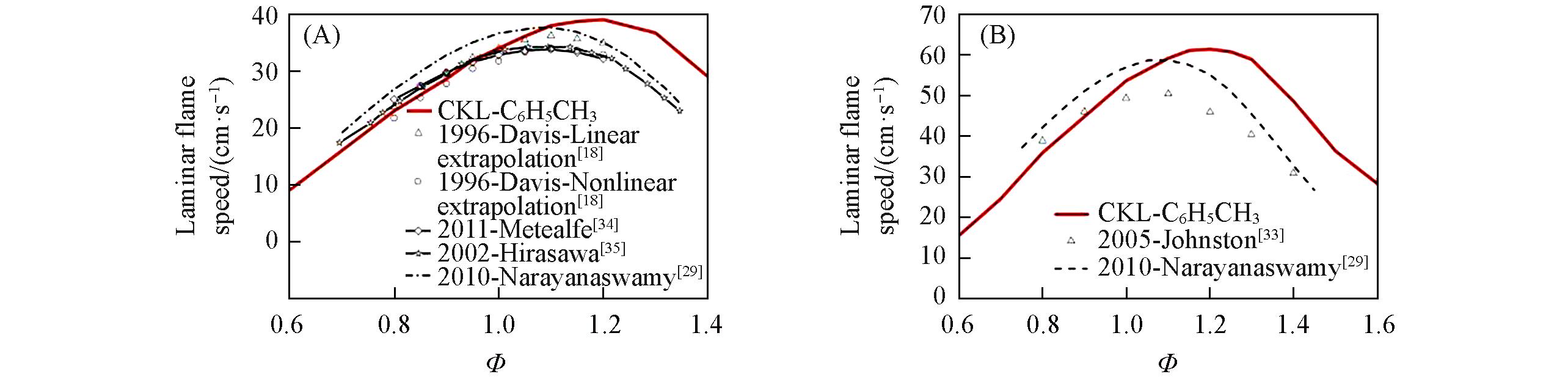
Fig.7 Laminar flame speed for toluene/air mixtures predicted by different mechanisms compared with experimental data(A) p=0.101 MPa, T=298 K; (B) p=0.304 MPa, T=450 K.
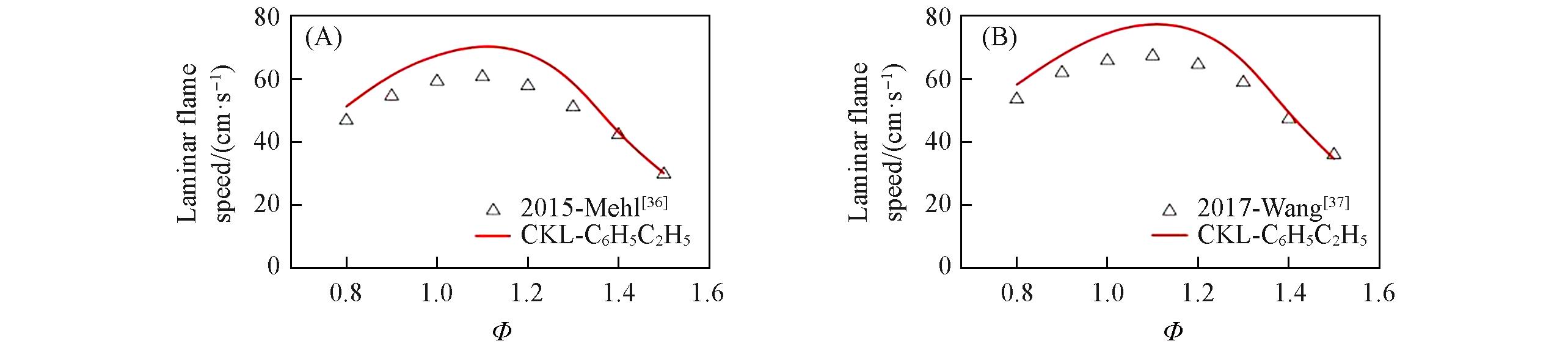
Fig.8 Laminar flame speed for ethylbenzene/air mixtures predicted by different mechanism compared with experimental data(A) p=0.101 MPa, T=398 K; (B) p=0.101 MPa, T=423 K.
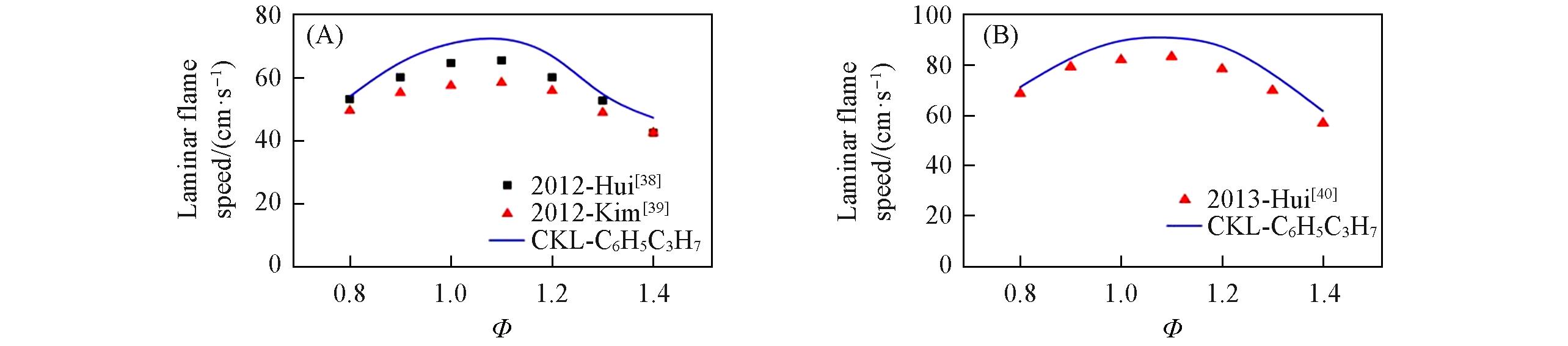
Fig.9 Laminar flame speed for n⁃propylbenzene/air mixtures predicted by different mechanism compared with experimental data(A) p=0.101 MPa, T=400 K; (B) p=0.101 MPa, T=470 K.
| 1 | Ning H. B., Li Z. R., Li X. Y., Acta Phys. Chim. Sin., 2016, 32(1), 131—153 |
| 甯红波, 李泽荣, 李象远. 物理化学学报, 2016, 32(1), 131—153 | |
| 2 | Zhou C. W., Li Y., Burke U., Banyon C., Somers K. P., Ding S., Khan S., Hargis J. W., Sikes T., Mathieu O., Petersen E. L., Alabbad M., Farooq A., Pan Y. S., Zhang Y. J., Huang Z. H., Lopea J., Loparo Z., Vasu S. S., Curran H. J., Combust. Flame, 2018, 197, 423—438 |
| 3 | Wang H., You X. Q., Joshi A. V., Davis S. G., Laskin A., Egolfopoulos F., Law C. K., USC Mech Version II, High⁃temperature Combustion Reaction Model of H2/CO/C1—C4 Compounds, http://ignis.usc.edu/USC_Mech_II.htm |
| 4 | Mechanical and Aerospace Engineering(Combustion Research), San Diego Mechanism, University of California at San Diego, 2016, http://combustion.ucsd.edu |
| 5 | Smith G. P., Golden D. M., Frenklach M., Moriarty N. W., Eitener B., Goldenberg M., Bowman C. T., Hanson R. K., Song S., Gardiner W. C. Jr., Lissianski V. V., Qin Z. W., GRI⁃Mech 3.0, http://www.me.berkeley.edu/gri_mech/ |
| 6 | Li X. Y., Shentu J. T., Li Y. W., Li J. Q., Wang J. B., Chem. J. Chinese Universities, 2020, 41(4), 772—779 |
| 李象远, 申屠江涛, 李宜蔚, 李娟琴, 王静波. 高等学校化学学报, 2020, 41(4), 772—779 | |
| 7 | Li Y. W., Shentu J. T., Wang J. B., Li X. Y., Chem. J. Chinese Universities, 2021, 42(6), 1871—1880 |
| 李宜蔚, 申屠江涛, 王静波, 李象远. 高等学校化学学报, 2021, 42(6), 1871—1880 | |
| 8 | Wang H., Sheen D. A., Prog. Energy Combust. Sci., 2015, 47, 1—31 |
| 9 | Denbign K. G., Principles of Chemical Equilibrium: with Applications to Chemistry and Chemical Engineering(Fourth Edition), Cambridge University Press, Cambridge, 1981, 169 |
| 10 | Weltin E., J. Chem. Educ., 1994, 71(4), 295—297 |
| 11 | Ren H. S., Wang J. B., Li X. Y., Combustion Dynamics, Sichuan, CDS1.0, Center for Combustion Dynamics, Sichuan University, 2021.9, http://cds.scu.edu.cn/ |
| 12 | Vourliotakis G., Skevis G., Founti M. A., Energy Fuels, 2011, 25, 1950—1963 |
| 13 | Ranzi E., Frassoldati A., Stagni A., Pelucchi M., Cuoci A., Faravelli T., Int. J. Chem. Kinet., 2014, 46, 512—542 |
| 14 | Pelucchi M., Cavallotti C., Faravelli T., Klippenstein S. J., PhysChemChemPhys, 2018, 20, 10607—10627 |
| 15 | Pelucchi M., Bissoli M., Cavallotti C., Cuoci A., Faravelli T., Frassoldati A., Ranzi E., Stagni A., Energy Fuels, 2014, 28, 7178—7193 |
| 16 | Pelucchi M., Cavallotti C., Ranzi E., Frassoldati A., Faravelli T., Energy Fuels, 2016, 30, 8665—8679 |
| 17 | Darcy D., Nakamura H., Tobin C. J., Mehl M., Metcalfe W. K., Pitz W. J., Westbrook C. K., Curran H. J., Combust. Flame, 2014, 161(1), 65—74 |
| 18 | Davis S. G., Law C. K., Combust. Sci. Technol., 1998, 140, 427—449 |
| 19 | Andrae J. C. G., Björnbom P., Cracknel R. F., Kalghatgi G. T., Combust. Flame, 2007, 149, 2—24 |
| 20 | Tan N. X., Wang J. B., Hua X. X., Li Z. R., Li X. Y., Chem. J. Chinese Universities, 2011, 32(8), 1832—1837 |
| 谈宁馨, 王静波, 华晓筱, 李泽荣, 李象远.高等学校化学学报, 2011, 32(8), 1832—1837 | |
| 21 | Guo J. J., Tang S. Y., Li R., Tan N. X., Acta Phys. Chim. Sin., 2019, 35(2), 182—192 |
| 郭俊江, 唐石云, 李瑞, 谈宁馨. 物理化学学报, 2019, 35(2), 182—192 | |
| 22 | Chemkin⁃Pro, Reaction Design, San Diego, 2010 |
| 23 | Burcat A., Snyder C., Brabbs T., Ignition Delay Times of Benzene and Toluene with Oxygen in Argon Mixtures, NASA Technical Memorandum 87312, Lewis Research Center, Cleveland Ohio, 1986 |
| 24 | Saggese C., Frassoldati A., Cuoci A., Faravelli T., Ranzi E., Combust. Flame, 2013, 160, 1168—1190 |
| 25 | Fieweger K., Blumenthal R., Adomeit G., Shock⁃tube Investigations on the Self⁃ignition of Hydrocarbon⁃air Mixtures at High Pressures, Twenty⁃fifth Symposium(International) on Combustion, University of California at Irvine, CA, 1994, 1579—1585 |
| 26 | Bounaceur R., Da Costa I., Fournet R., Billaud F., Battin⁃Leclerc F., Int. J. Chem. Kinet., 2005, 37, 25—49 |
| 27 | Sakai Y., Inamura T., Ogura T., Koshi M., Pitz W. J., Jsae/sae International Fuels & Lubricants Meeting, SAE International,Tokyo, 2007 |
| 28 | Zhang C. H., Li P., Guo J. J., Li X. Y., Energy Fuels, 2012, 26, 1107—1113 |
| 29 | Narayanaswamy K., Blanquart G., Pitsch H., Combust. Flame, 2010, 157, 1879—1898 |
| 30 | Sakai Y., Miyoshi A., Koshi M., Pitz W. J., Proc. Combust. Inst., 2009, 32, 411—418 |
| 31 | Andrae J.C.G., Brinck T., Kalghatgi G.T., Combust. Flame, 2008, 155, 696—712 |
| 32 | Shen H., Oehlschlaeger M. A., Combust. Flame, 2009, 156(5), 1053—1062 |
| 33 | Johnston R. J., Farrell J. T., P. Combust. Inst., 2005, 30, 217—224 |
| 34 | Metcalfe W. K., Dooley S., Dryer F. L., Energy Fuels, 2011, 25, 4915—4936 |
| 35 | Hirasawa T., Sung C. J., Joshi A., Yang Z., Law C. K., Proc. Combust. Inst., 2002, 29, 1427—1434 |
| 36 | Mehl M., Herbinet O., Dirrenberger P., Bounaceur R., Glaude P. A., Battin⁃Leclerc F., Pitz W. J., Proc. Combust. Inst., 2015, 35, 341—348 |
| 37 | Wang G., Li Y., Yuan W., Zhou Z., Wang Y., Wang Z., Combust. Flame, 2017, 184, 312—323 |
| 38 | Hui X., Das A. K., Kumar K., Sung C. J., Dooley S., Dryer F. L., Fuel, 2012, 97, 695—702 |
| 39 | Kim H. H., Diévart P., Santner J., Dooley S. W., Ju Y. G., Measurements and Modeling of the Laminar Flame Speeds of n⁃Propyl and 1,3,5⁃Trimethyl Benzenes at Moderate Pressures, 50th AIAA Aerospace Sciences Meeting Including the New Horizons Forum and Aerospace Exposition, American Institute of Aeronautics and Astronautics, Nashville, 2012 |
| 40 | Hui X., Sung C. J., Fuel, 2013, 109, 191—200 |
| [1] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| [2] | 李宜蔚, 申屠江涛, 王静波, 李象远. 燃烧反应机理构建的极小反应网络方法: C1燃料燃烧[J]. 高等学校化学学报, 2021, 42(6): 1871. |
| [3] | 李象远, 申屠江涛, 李宜蔚, 李娟琴, 王静波. 燃烧反应机理构建的极小反应网络方法 |
| [4] | 李象远,姚晓霞,申屠江涛,孙晓慧,李娟琴,刘明夏,许诗敏. 燃烧反应机理构建的双参数速率常数方法[J]. 高等学校化学学报, 2020, 41(3): 512. |
| [5] | 于环洋, 崔滢, 刘贤英, 史作森. 磺酰化修饰聚苯乙烯的合成及芳香烃萃取性能[J]. 高等学校化学学报, 2014, 35(1): 186. |
| [6] | 彭璇, 汪文川 . 狭缝孔内甲烷蒸汽重整化学平衡的分子模拟[J]. 高等学校化学学报, 2006, 27(8): 1530. |
| [7] | 熊裕堂, 胡永钢, 夏炽中. 对-十二烷氧基二硫代苯甲酸镍(Ⅱ)配合物液晶型色谱固定相的研制[J]. 高等学校化学学报, 2000, 21(9): 1413. |
| [8] | 余艺华, 袁直, 何炳林, 顾汉卿. 胆红素-白蛋白复合物的化学平衡离解常数及结合自由能的测定[J]. 高等学校化学学报, 1997, 18(4): 661. |
| [9] | 张贵珠, 高志, 何锡文, 史慧明. 含表面活性剂多元配合物体系中化学平衡的研究[J]. 高等学校化学学报, 1991, 12(8): 1018. |
| [10] | 李志良, 李梦龙, 石乐明, 俞汝勤. 卡尔曼滤波光谱法用于化学平衡研究[J]. 高等学校化学学报, 1990, 11(8): 825. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||