

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (7): 1192.doi: 10.7503/cjcu20170042
赵齐齐, 梁苗苗, 马洋洋, 李晓凯, 朱华结( ), 李婉(
), 李婉( )
)
收稿日期:2017-01-18
出版日期:2017-07-10
发布日期:2017-05-18
作者简介:联系人简介: 朱华结, 男, 博士, 教授, 博士生导师, 主要从事手性药物化学研究. E-mail:基金资助:
ZHAO Qiqi, LIANG Miaomiao, MA Yangyang, LI Xiaokai, ZHU Huajie*( ), LI Wan*(
), LI Wan*( )
)
Received:2017-01-18
Online:2017-07-10
Published:2017-05-18
Contact:
ZHU Huajie,LI Wan
E-mail:zhuhuajie@hotmail.com;liwanjingmin@163.com
摘要:
以L-色氨酸为原料合成了5个伯酰胺结构的轴手性双咔啉N—O催化剂N2,N2'-二氧-9,9'-二甲基-3,3'-取代甲酰胺-β-双咔啉(4A~4E), 并用于不对称催化酮亚胺的还原反应. 结果表明, 催化剂的催化转化率较高(80%~98%), 立体选择性(e. e.值)较好, 其中催化剂N2,N2'-二氧-9,9'-二甲基-3,3'-环己基甲酰胺-β-双咔啉(4B)的催化转化率达到了98%, e. e.值达68%.
中图分类号:
TrendMD:
赵齐齐, 梁苗苗, 马洋洋, 李晓凯, 朱华结, 李婉. 新轴手性N,N-Dioxide Biscarbolin衍生物催化酮亚胺的不对称硅氢化反应. 高等学校化学学报, 2017, 38(7): 1192.
ZHAO Qiqi, LIANG Miaomiao, MA Yangyang, LI Xiaokai, ZHU Huajie, LI Wan. New Chiral Biscarboline N,N-Dioxide Derivatives as Catalysts over Enantioselective Hydrosilylation of Keteoimines†. Chem. J. Chinese Universities, 2017, 38(7): 1192.
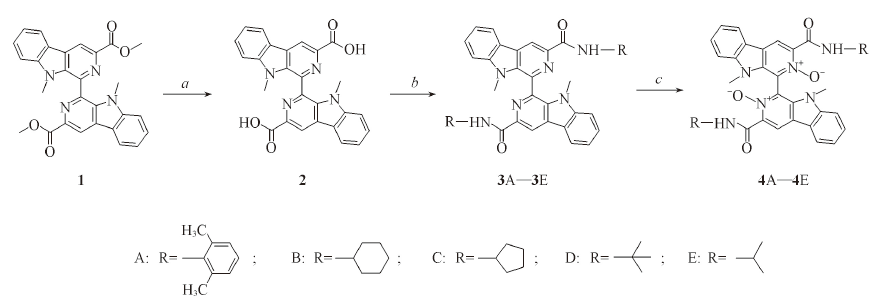
Scheme 1 Synthetic routes of chiral ligands 4A—4EReaction conditions: a. NaOH, H2O, MeOH, 60 ℃; b. (i) isobutyl-chloroformate(2.4 mmol), Et3N(2.4 mmol), DCM, 0 ℃, 20 min; (ii) amine(2.2 mmol), r. t., 12 h; c. DCM, m-CPBA(8 mmol), r. t., 24 h.
| Compd. | Appearance | Yield(%) | Elemental analysis(%, cacld. ) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 3A | White solid | 85 | 76.85(76.81) | 5.58(5.52) | 12.84(12.80) |
| 3B | White solid | 90 | 74.50(74.48) | 6.55(6.58) | 13.74(13.71) |
| 3C | White solid | 90 | 73.92(73.95) | 6.23(6.21) | 14.33(14.37) |
| 3D | White solid | 90 | 72.85(72.83) | 6.51(6.47) | 14.95(14.99) |
| 3E | White solid | 90 | 72.14(72.16) | 6.08(6.06) | 15.74(15.78) |
| 4A | White solid | 40 | 73.26(73.24) | 5.68(5.72) | 12.23(12.20) |
| 4B | White solid | 43 | 70.72(70.79) | 6.23(6.25) | 13.06(13.03) |
| 4C | White solid | 40 | 70.16(70.11) | 5.81(5.88) | 13.60(13.63) |
| 4D | White solid | 45 | 68.85(68.90) | 6.18(6.12) | 14.20(14.18) |
| 4E | White solid | 45 | 68.03(68.07) | 5.68(5.71) | 14.88(14.88) |
Table 1 Appearance, yields and elemental analysis data of compounds 3A—3E and 4A—4E
| Compd. | Appearance | Yield(%) | Elemental analysis(%, cacld. ) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 3A | White solid | 85 | 76.85(76.81) | 5.58(5.52) | 12.84(12.80) |
| 3B | White solid | 90 | 74.50(74.48) | 6.55(6.58) | 13.74(13.71) |
| 3C | White solid | 90 | 73.92(73.95) | 6.23(6.21) | 14.33(14.37) |
| 3D | White solid | 90 | 72.85(72.83) | 6.51(6.47) | 14.95(14.99) |
| 3E | White solid | 90 | 72.14(72.16) | 6.08(6.06) | 15.74(15.78) |
| 4A | White solid | 40 | 73.26(73.24) | 5.68(5.72) | 12.23(12.20) |
| 4B | White solid | 43 | 70.72(70.79) | 6.23(6.25) | 13.06(13.03) |
| 4C | White solid | 40 | 70.16(70.11) | 5.81(5.88) | 13.60(13.63) |
| 4D | White solid | 45 | 68.85(68.90) | 6.18(6.12) | 14.20(14.18) |
| 4E | White solid | 45 | 68.03(68.07) | 5.68(5.71) | 14.88(14.88) |
| Compd. | 1H NMR(CDCl3, 600 MHz), δ | 13C NMR(CDCl3, 150 MHz), δ |
|---|---|---|
| 3A | 9.44—9.36(m, 2H), 9.25(t, J=5.7 Hz, 2H), 8.33(d, J=7.9 Hz, 2H), 7.70(t, J=7.7 Hz, 2H), 7.50(d, J=8.4 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.30(t, J=7.6 Hz, 2H), 7.19(d, J=7.7 Hz, 4H), 3.54—3.47(m, 6H), 1.19(d, J=6.7 Hz, 12H) | 164.42, 146.14, 143.18, 138.73, 138.45, 137.44, 131.55, 131.44, 129.53, 128.17, 123.44, 122.25, 121.54, 121.19, 115.37, 109.83, 77.24, 77.03, 76.82, 32.39, 29.01, 23.78, 23.60 |
| 3B | 9.13(s, 2H), 8.36(s, 2H), 7.91(s, 2H), 7.68(s, 2H), 7.47(s, 2H), 7.43(t, J=7.5 Hz, 2H), 4.02(s, 2H), 3.31(s, 6H), 2.02(s, 4H), 1.69(s, 4H), 1.61(d, J=13.1 Hz, 2H), 1.44—1.37(m, 4H), 1.21(s, 4H), 1.12(d, J=30.4 Hz, 2H) | 164.11, 143.00, 139.05, 138.35, 137.13, 131.07, 129.23, 122.16, 121.50, 120.87, 114.72, 109.89, 48.38, 33.30, 32.28, 25.55, 25.08 |
| 3C | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.94(d, J=8.0 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.49—4.43(m, 2H), 3.29(s, 6H), 2.07(dt, J=13.0, 5.6 Hz, 4H), 1.68—1.63(m, 4H), 1.63—1.57(m, 4H), 1.48(dt, J=12.4, 6.2 Hz, 4H) | 164.70, 142.99, 138.99, 138.38, 137.13, 131.01, 129.26, 122.15, 121.49, 120.90, 114.69, 109.90, 51.18, 33.26, 32.22, 23.87 |
| 3D | 9.12(s, 2H), 8.32(d, J=7.9 Hz, 2H), 7.95(s, 2H), 7.68(t, J=7.6 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 3.28(d, J=8.8 Hz, 6H), 1.47(s, 18H) | 164.42, 143.00, 139.71, 138.19, 137.11, 131.07, 129.22, 122.08, 121.50, 120.85, 114.23, 109.87, 51.00, 32.18, 28.97 |
| 3E | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.85(d, J=8.3 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.47(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.35(dq, J=13.4, 6.6 Hz, 2H), 3.29(d, J=8.7 Hz, 6H), 1.24(t, J=6.1 Hz, 12H) | 164.25, 142.98, 139.00, 138.37, 137.13, 131.00, 129.26, 122.15, 121.47, 120.90, 114.71, 109.90, 41.45, 32.18, 22.81 |
| 4A | 12.68(s, 2H), 9.45(d, J=1.0 Hz, 2H), 8.25(d, J=7.9 Hz, 2H), 7.66(t, J=7.8 Hz, 2H), 7.45(dd, J=12.1, 6.4 Hz, 4H), 7.13—7.08(m, 6H), 3.40(d, J=0.6 Hz, 6H), 2.31(s, 12H) | 158.85, 143.93, 138.89, 134.79, 134.05, 132.99, 129.68, 129.32, 128.09, 127.02, 125.24, 122.96, 122.33, 121.86, 121.40, 109.97, 29.31, 18.83 |
| 4B | 11.07(d, J=7.8 Hz, 2H), 9.34(s, 2H), 8.22(d, J=7.9 Hz, 2H), 7.64—7.59(m, 2H), 7.42(t, J=7.5 Hz, 2H), 7.38(d, J=8.3 Hz, 2H), 4.08—4.00(m, 2H), 3.25(s, 6H), 2.02(dd, J=16.6, 13.2 Hz, 4H), 1.72(dd, J=13.1, 9.5 Hz, 6H), 1.60(dd, J=9.3, 3.9 Hz, 2H), 1.40(dd, J=18.5, 5.6 Hz, 4H), 1.22—1.11(m, 4H) | 159.61, 143.85, 138.52, 133.19, 129.05, 125.25, 122.63, 122.04, 121.73, 121.39, 120.89, 109.83, 48.92, 32.89, 29.48, 25.64, 24.89 |
| 4C | 11.03(t, J=14.7 Hz, 2H), 9.27(s, 2H), 8.14(d, J=7.9 Hz, 2H), 7.54(t, J=7.7 Hz, 2H), 7.34(t, J=7.5 Hz, 2H), 7.30(d, J=8.3 Hz, 2H), 4.37(dd, J=13.7, 6.9 Hz, 2H), 3.17(s, 6H), 2.00(dd, J=11.6, 4.9 Hz, 4H), 1.62(d, J=3.1 Hz, 4H), 1.51(dd, J=24.0, 13.2 Hz, 8H) | 159.12, 142.85, 137.51, 132.11, 128.06, 124.21, 121.64, 121.05, 120.70, 120.39, 119.83, 108.82, 50.58, 31.95, 28.50, 23.00 |
| 4D | 11.14(s, 2H), 9.36(d, J=1.0 Hz, 2H), 8.19(d, J=7.9 Hz, 2H), 7.63(t, J=7.7 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.39(d, J=8.2 Hz, 2H), 3.25(d, J=0.5 Hz, 6H), 1.51(s, 18H) | 159.38, 143.85, 138.51, 133.80, 129.01, 125.22, 122.63, 122.02, 121.60, 121.40, 120.51, 109.81, 51.49, 29.51, 28.77 |
| 4E | 11.00(d, J=7.5 Hz, 2H), 9.35(s, 2H), 8.21(d, J=7.9 Hz, 2H), 7.61(dd, J=11.4, 4.1 Hz, 2H), 7.42(t, J=7.5 Hz, 2H), 7.37(d, J=8.3 Hz, 2H), 4.33(dd, J=13.8, 6.7 Hz, 2H), 3.24(s, 6H), 1.27(dd, J=6.6, 3.2 Hz, 12H) | 159.69, 143.87, 138.51, 133.17, 129.10, 125.23, 122.68, 122.09, 121.71, 121.39, 120.88, 109.83, 41.99, 29.54, 22.61 |
Table 2 1H NMR and 13C NMR data of compounds 3A—3E and 4A—4E
| Compd. | 1H NMR(CDCl3, 600 MHz), δ | 13C NMR(CDCl3, 150 MHz), δ |
|---|---|---|
| 3A | 9.44—9.36(m, 2H), 9.25(t, J=5.7 Hz, 2H), 8.33(d, J=7.9 Hz, 2H), 7.70(t, J=7.7 Hz, 2H), 7.50(d, J=8.4 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.30(t, J=7.6 Hz, 2H), 7.19(d, J=7.7 Hz, 4H), 3.54—3.47(m, 6H), 1.19(d, J=6.7 Hz, 12H) | 164.42, 146.14, 143.18, 138.73, 138.45, 137.44, 131.55, 131.44, 129.53, 128.17, 123.44, 122.25, 121.54, 121.19, 115.37, 109.83, 77.24, 77.03, 76.82, 32.39, 29.01, 23.78, 23.60 |
| 3B | 9.13(s, 2H), 8.36(s, 2H), 7.91(s, 2H), 7.68(s, 2H), 7.47(s, 2H), 7.43(t, J=7.5 Hz, 2H), 4.02(s, 2H), 3.31(s, 6H), 2.02(s, 4H), 1.69(s, 4H), 1.61(d, J=13.1 Hz, 2H), 1.44—1.37(m, 4H), 1.21(s, 4H), 1.12(d, J=30.4 Hz, 2H) | 164.11, 143.00, 139.05, 138.35, 137.13, 131.07, 129.23, 122.16, 121.50, 120.87, 114.72, 109.89, 48.38, 33.30, 32.28, 25.55, 25.08 |
| 3C | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.94(d, J=8.0 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.49—4.43(m, 2H), 3.29(s, 6H), 2.07(dt, J=13.0, 5.6 Hz, 4H), 1.68—1.63(m, 4H), 1.63—1.57(m, 4H), 1.48(dt, J=12.4, 6.2 Hz, 4H) | 164.70, 142.99, 138.99, 138.38, 137.13, 131.01, 129.26, 122.15, 121.49, 120.90, 114.69, 109.90, 51.18, 33.26, 32.22, 23.87 |
| 3D | 9.12(s, 2H), 8.32(d, J=7.9 Hz, 2H), 7.95(s, 2H), 7.68(t, J=7.6 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 3.28(d, J=8.8 Hz, 6H), 1.47(s, 18H) | 164.42, 143.00, 139.71, 138.19, 137.11, 131.07, 129.22, 122.08, 121.50, 120.85, 114.23, 109.87, 51.00, 32.18, 28.97 |
| 3E | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.85(d, J=8.3 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.47(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.35(dq, J=13.4, 6.6 Hz, 2H), 3.29(d, J=8.7 Hz, 6H), 1.24(t, J=6.1 Hz, 12H) | 164.25, 142.98, 139.00, 138.37, 137.13, 131.00, 129.26, 122.15, 121.47, 120.90, 114.71, 109.90, 41.45, 32.18, 22.81 |
| 4A | 12.68(s, 2H), 9.45(d, J=1.0 Hz, 2H), 8.25(d, J=7.9 Hz, 2H), 7.66(t, J=7.8 Hz, 2H), 7.45(dd, J=12.1, 6.4 Hz, 4H), 7.13—7.08(m, 6H), 3.40(d, J=0.6 Hz, 6H), 2.31(s, 12H) | 158.85, 143.93, 138.89, 134.79, 134.05, 132.99, 129.68, 129.32, 128.09, 127.02, 125.24, 122.96, 122.33, 121.86, 121.40, 109.97, 29.31, 18.83 |
| 4B | 11.07(d, J=7.8 Hz, 2H), 9.34(s, 2H), 8.22(d, J=7.9 Hz, 2H), 7.64—7.59(m, 2H), 7.42(t, J=7.5 Hz, 2H), 7.38(d, J=8.3 Hz, 2H), 4.08—4.00(m, 2H), 3.25(s, 6H), 2.02(dd, J=16.6, 13.2 Hz, 4H), 1.72(dd, J=13.1, 9.5 Hz, 6H), 1.60(dd, J=9.3, 3.9 Hz, 2H), 1.40(dd, J=18.5, 5.6 Hz, 4H), 1.22—1.11(m, 4H) | 159.61, 143.85, 138.52, 133.19, 129.05, 125.25, 122.63, 122.04, 121.73, 121.39, 120.89, 109.83, 48.92, 32.89, 29.48, 25.64, 24.89 |
| 4C | 11.03(t, J=14.7 Hz, 2H), 9.27(s, 2H), 8.14(d, J=7.9 Hz, 2H), 7.54(t, J=7.7 Hz, 2H), 7.34(t, J=7.5 Hz, 2H), 7.30(d, J=8.3 Hz, 2H), 4.37(dd, J=13.7, 6.9 Hz, 2H), 3.17(s, 6H), 2.00(dd, J=11.6, 4.9 Hz, 4H), 1.62(d, J=3.1 Hz, 4H), 1.51(dd, J=24.0, 13.2 Hz, 8H) | 159.12, 142.85, 137.51, 132.11, 128.06, 124.21, 121.64, 121.05, 120.70, 120.39, 119.83, 108.82, 50.58, 31.95, 28.50, 23.00 |
| 4D | 11.14(s, 2H), 9.36(d, J=1.0 Hz, 2H), 8.19(d, J=7.9 Hz, 2H), 7.63(t, J=7.7 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.39(d, J=8.2 Hz, 2H), 3.25(d, J=0.5 Hz, 6H), 1.51(s, 18H) | 159.38, 143.85, 138.51, 133.80, 129.01, 125.22, 122.63, 122.02, 121.60, 121.40, 120.51, 109.81, 51.49, 29.51, 28.77 |
| 4E | 11.00(d, J=7.5 Hz, 2H), 9.35(s, 2H), 8.21(d, J=7.9 Hz, 2H), 7.61(dd, J=11.4, 4.1 Hz, 2H), 7.42(t, J=7.5 Hz, 2H), 7.37(d, J=8.3 Hz, 2H), 4.33(dd, J=13.8, 6.7 Hz, 2H), 3.24(s, 6H), 1.27(dd, J=6.6, 3.2 Hz, 12H) | 159.69, 143.87, 138.51, 133.17, 129.10, 125.23, 122.68, 122.09, 121.71, 121.39, 120.88, 109.83, 41.99, 29.54, 22.61 |
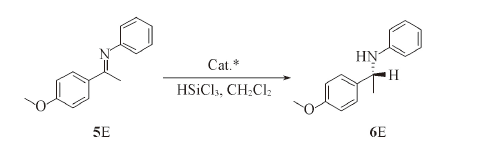
Scheme 2 Enantioselective hydrosilylation of ketoimine 5E catalyzed by compounds 4A—4EAll the reactions were performed using compound 5E(0.1 mmol) and HSiCl3(0.2 mmol) in the presence of compound 4 at -20 ℃ for 16 h.
| Cat. | Yield(%) | e. e.(%) | Cat. | Yield(%) | e. e.(%) |
|---|---|---|---|---|---|
| 4A | 80 | 40 | 4D | 95 | 19 |
| 4B | 98 | 50 | 4E | 90 | 21 |
| 4C | 90 | 35 |
Table 3 Enantioselective hydrosilylation of ketoimine 5E catalyzed by compounds 4A—4E*
| Cat. | Yield(%) | e. e.(%) | Cat. | Yield(%) | e. e.(%) |
|---|---|---|---|---|---|
| 4A | 80 | 40 | 4D | 95 | 19 |
| 4B | 98 | 50 | 4E | 90 | 21 |
| 4C | 90 | 35 |
| Compd. | R1 | R2 | Yield(%) | e. e.(%) |
|---|---|---|---|---|
| 5A | H | H | 95 | 57 |
| 5B | H | MeO | 90 | 68 |
| 5C | H | Br | 85 | 45 |
| 5B | NO2 | H | 93 | 40 |
| 5E | MeO | H | 98 | 63 |
Table 4 Enantioselective hydrosilylation of ketoimines 5A—5E catalyzed by catalyst 4B
| Compd. | R1 | R2 | Yield(%) | e. e.(%) |
|---|---|---|---|---|
| 5A | H | H | 95 | 57 |
| 5B | H | MeO | 90 | 68 |
| 5C | H | Br | 85 | 45 |
| 5B | NO2 | H | 93 | 40 |
| 5E | MeO | H | 98 | 63 |
| [1] | Jones S., Warner C., Org. Biomol. Chem., 2012, 10, 2189—2200 |
| [2] | Wang Z. Y., Ye X. X., Wei S. Y., Wu P. C., Zhang A. J., Sun J., Org. Lett., 2006, 8, 999—1001 |
| [3] | Wang Z. Y., Wang C., Zhou L., Sun J., Org. Lett., 2013, 11, 787—797 |
| [4] | Feringa B. L., Van Delden R. A., Angew. Chem. Int. Ed., 1999, 38, 3418—3428 |
| [5] | Jang H., Liu W., Zhang S. X., Liao W. W., Chem. Res. Chinese Universities,2016, 32(2), 385—389 |
| [6] | Blaser H. U., Malan C., Pugin B., Spindler F., Studer M., Adv. Synth. Catal., 2003, 345, 103—151 |
| [7] | Pan W., Deng Y., He J. B., Bai B., Zhu H. J., Tetrahedron,2013, 69, 7253—7257 |
| [8] | Kobayashi S., Ishitani H., Chem. Rev., 1999, 99, 1069—1094 |
| [9] | Yu Z., Jin W., Jiang Q., Angew. Chem. Int. Ed., 2012, 51, 6060—6072 |
| [10] | Xie J. H., Zhu S. F., Zhou Q. L., Chem. Soc.Rev. ,2012, 41, 4126—4139 |
| [11] | Cobley C. J., Henschke J. P., Adv. Synth. Catal., 2003, 345, 195—201 |
| [12] | Noyor R., Hashiguchi S., Acc. Chem. Res., 1997, 30, 97—102 |
| [13] | Moessner C., Bolm C., Angew. Chem. Int. Ed., 2006, 117, 7736—7739 |
| [14] | Kobayashi S., Yasuda M., Hachiya L., Chem. Lett., 1996, 5, 407—408 |
| [15] | Iwasaki F., Onomura O., Mishima K., Kanematsu T., Maki T., Matsumura Y., Tetrahedron Lett., 2001, 42, 2525—2527 |
| [16] | Baudequin C., Chaturvedi D., Tsoggoeva S. B., Eur. J. Org. Chem., 2007, 16, 2623—2629 |
| [17] | Zhang Z., Rooshenas P., Hausmann H., Schreiner P. R., Synthesis,2009, 9, 1531—1539 |
| [18] | Kanemitsu T., Umehara A., HaneJi R., Nagata K., Itoh T., Tetrahedron,2012, 20, 3893—3898 |
| [19] | Bai B., Shen L., Ren J., Zhu H. J., Adv. Synth. Catal., 2012, 354, 354—358 |
| [20] | Shen Y. C., Li Y. X., Wen X. J., Feng X. M., Chin. J. Org. Chem., 2005, 25, 272—283 |
| (申永存, 李燕霞, 闻雪敬, 冯小明. 有机化学, 2005, 25, 272—283) | |
| [21] | Liu X. H., Lin L. L., Feng X. M., Org. Chem. Front., 2014, 3, 298—302 |
| [22] | Deng Y., Pan W., Pei Y. N., Li J. L., Bai B., Zhu H. J., Tetrahedron,2013, 68, 10431—10437 |
| [23] | Tang W., Zhang X., Chem. Rev., 2003, 103, 3029—3069 |
| [24] | Feng J., Lin L., Yu K., Liu X., Feng X. M., Adv. Synth. Catal., 2015, 357, 1305—1310 |
| [25] | Feng J., Fu X., Chen Z., Lin L., Liu X., Feng X. M., Org. Lett. ,2013, 15, 2640—2643 |
| [26] | Liu X. H., Lin L. L., Feng X. M., Acc. Chem. Res., 2011, 44, 574—587 |
| [27] | Liu L., Yang Q., Yu H., Li J. L., Pei Y. N., Zhu H. J., Tetrahedron,2015, 71, 3296—3302 |
| [28] | Pei Y. N., Deng Y., Li J. L., Liu L., Zhu H. J., Tetrahedron Lett., 2014, 55, 2948—2952 |
| [29] | Pan W., Ma W. G., Yang X. D., Zheng Y. H., Song B. Q., Niu Y. Z., Gu J., Hu D. B., Yang Q., Zhu H. J., Chem. J. Chinese Universites,2015, 36(2), 325—329 |
| (潘威, 马文广, 杨晓东, 郑昀晔, 宋碧清, 牛永志, 古吉, 胡栋宝, 杨芹, 朱华结.高等学校化学学报, 2015, 36(2), 325—329) |
| [1] | 黄秋红, 李文军, 李鑫. 有机催化靛红衍生酮亚胺与噁唑酮的不对称Mannich型加成反应[J]. 高等学校化学学报, 2022, 43(8): 20220131. |
| [2] | 朱洁莲, 夏晓峰, 梁敏婷, 刘湘, 李和兴. CuFe2O4纳米粒子催化芳香酮的不对称硅氢化反应[J]. 高等学校化学学报, 2016, 37(3): 539. |
| [3] | 黄庆东, 李春兰, 张宇, 崔立山, 朱百春, 义建军, 刘林, 郝海军. 桥连双(酮亚胺)和双(二酮亚胺)及其铝配合物的合成与催化活性[J]. 高等学校化学学报, 2014, 35(3): 524. |
| [4] | 姚金水, 吴佑实. 铑(I)催化的不对称硅氢化反应合成手性2-氨基-1-芳基乙醇研究[J]. 高等学校化学学报, 2002, 23(1): 68. |
| [5] | 徐国华, Higashitani Ko. OTS自组装单分子膜在玻璃表面形成过程的AFM研究[J]. 高等学校化学学报, 2000, 21(8): 1257. |
| [6] | 冯云龙, 刘世雄. 两个异亚硝基乙酰丙酮-N-芳基亚胺Pd(Ⅱ)配合物的合成、谱学性质和PdCl(C6H5—IAI)(C6H5NH2)的晶体结构[J]. 高等学校化学学报, 2000, 21(10): 1455. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||