

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (11): 2257.doi: 10.7503/cjcu20190334
• Artickes:Chemical Biology • Previous Articles Next Articles
YE Xuwen,ZHANG Wenlong,WANG Zhijun,ZHAO Yaqin,YANG Binsheng( )
)
Received:2019-06-14
Online:2019-11-10
Published:2019-10-15
Contact:
YANG Binsheng
E-mail:yangbs@sxu.edu.cn
Supported by:CLC Number:
TrendMD:
YE Xuwen,ZHANG Wenlong,WANG Zhijun,ZHAO Yaqin,YANG Binsheng. Binding of Trifluoperazine to N-terminal Domain of Euplotes Octocarinatus Centrin and the Influence on Its Function †[J]. Chem. J. Chinese Universities, 2019, 40(11): 2257.
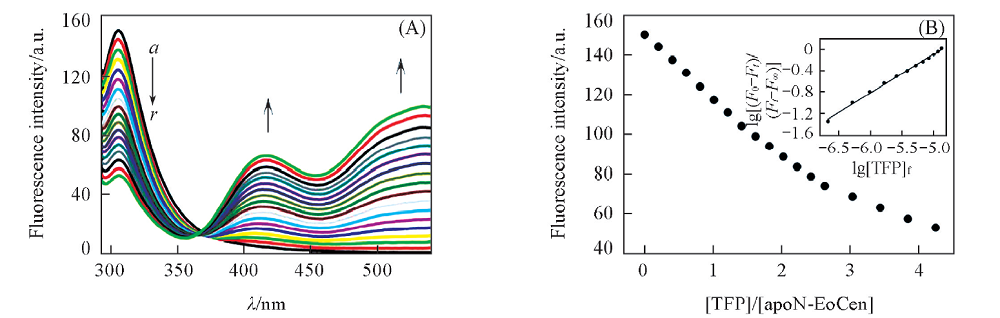
Fig.2 Fluorescence spectra produced by the addition of TFP(0.4 mmol/L) to apoN-EoCen(1.0×10-5 mol/L, 1 mL)(A) and titration curve of fluorescent intensity at 305 nm for apoN-EoCen against TFP/apoN-EoCen molar ratio in 10 mmol/L Hepes buffer(pH=7.4) solution(B) (A) Total volumes of TFP from spectrum a to r are 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 75, 85, 95, 105 μL, respectively; (B) inset: the fitting curve of lg[(F0-Fi)/(Fi-F∞)] vs. lg[TFP]f.
| Method | 10-3K/(L·mol-1) | n | ΔS/(J·mol-1) | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|---|---|
| ITC | 7.60±0.66 | 1 | 33.9 | -(12.26±0.49) | -22.54 |
| Fluorescence | 3.31±0.07 | 0.7 |
| Method | 10-3K/(L·mol-1) | n | ΔS/(J·mol-1) | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|---|---|
| ITC | 7.60±0.66 | 1 | 33.9 | -(12.26±0.49) | -22.54 |
| Fluorescence | 3.31±0.07 | 0.7 |
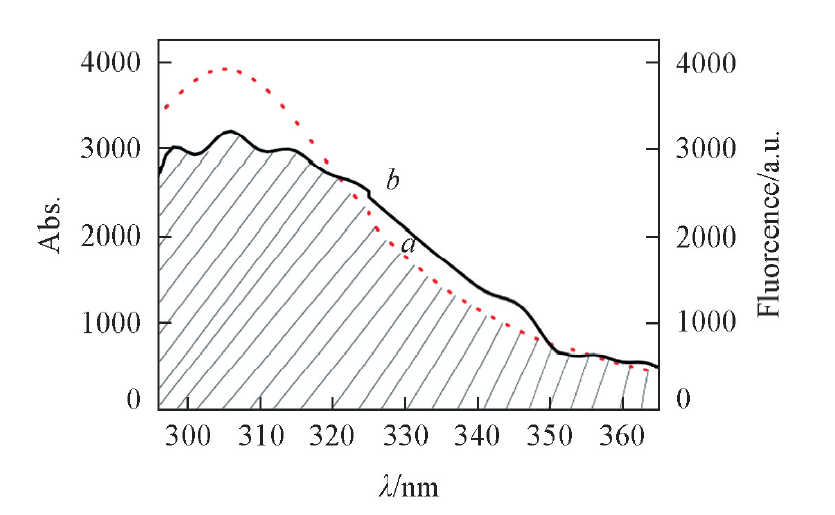
Fig.5 Overlap between fluorescence spectrum of apoN-EoCen(a) and absorption spectrum of TFP(b) The concentrations of TFP and apoN-EoCen both are 2.0×10-5 mol/L.
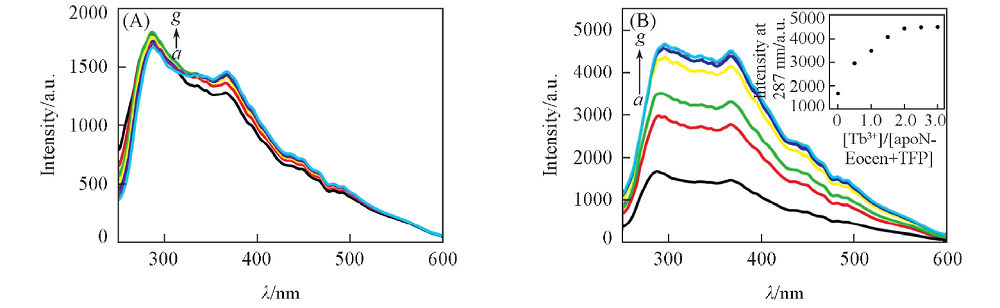
Fig.7 Resonance light scattering spectra of apoN-EoCen with the addition of TFP(A) and apoN-EoCen-TFP with the addition of Tb3+(B) in 10 mmol/L Hepes buffer solution(pH=7.4) Inset: titration curve of intensity at 287 nm against the concentration ratio of Tb3+ to apoN-EoCen-TFP. From spectum a to g the concentration ratios of TFP to apoN-EoCen or Tb3+ to apoN-EoCen-TFP are 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, respectively.
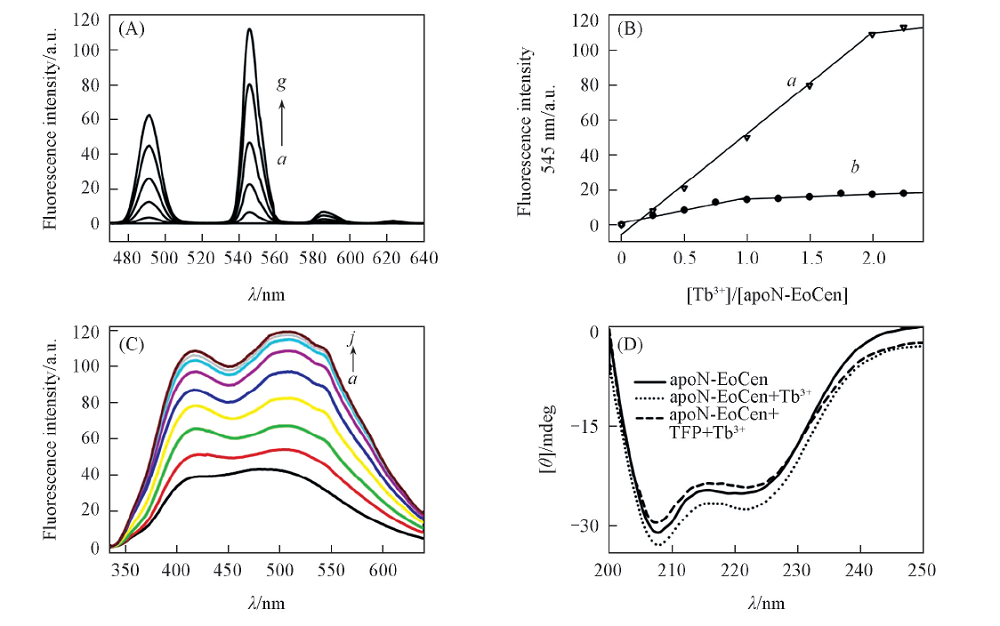
Fig.8 Tb3+ sensitized fluorescence spectra in the absence(A) and presence(C) of TFP, fluorescence intensity of Tb3+ at 545 nm as a function of Tb3+/apoN-EoCen ratio in the absence(a) and presence(b) of TFP(B), Far-UV CD spectra of apoN-EoCen, Tb2-N-EoCen and Tb2-N-EoCen-TFP(D) (A) Using 1 mmol/L Tb3+ solution to titrate the apoN-EoCen. Tb3+/N-EoCen ratio for spectrum a—g was 0, 0.25, 0.50, 1.00, 1.50, 2.00, 2.25, respectively. (B) The data of curve b are taken from the fluorescence intensity at 545 nm of spectra(C) and data of curve a are taken from spectra(A). (C) Using 1 mmol/L Tb3+ solution to titrate the apoN-EoCen-TFP. Tb3+/N-EoCen-TFP was 0, 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, 2.00, 2.25, respectively.
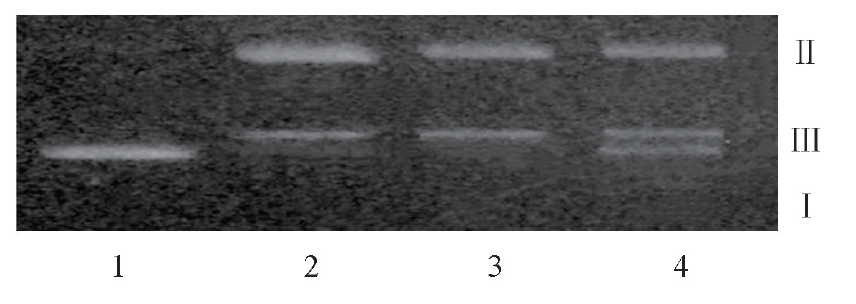
Fig.9 Gelatinous map of protein-cleaved pBR322 DNA in the presence of TFP Lane 1: pBR322DNA; lane 2: pBR322DNA+apoN-EoCen; lanes 3 and 4: on the basis of line 2, added 5, 10 times TFP. The concentrations of DNA, apoN-EoCen was 3.5×10-3 μg/μL and 14.8 μmol/L, respectively.
| [1] | Zhao Y.Q., Guo X. J., Yang B. S ., RSC Advances, 2017,7(17), 10206— 10214 |
| [2] | Liu W., Duan L., Sun T.J., Yang B. S ., BioMetals, 2016,29(6), 1047— 1058 |
| [3] | Shi E.X., Zhang W. L., Zhao Y. Q., Yang B. S ., RSC Advances, 2017,7(44), 27139— 27149 |
| [4] | Edington S.C., Gonzalez A., Middendorf T. R., Halling D. B., Aldrich R. W., Baiz C. R ., Proceedings of the National Academy of Sciences, 2018,115(14), E3126— E3143 |
| [5] | Shi E.X., Zhang W. L., Zhao Y. Q., Yang B. S ., Metallomics, 2017,9(12), 1796— 1808 |
| [6] | Rong Z.J., Zhao Y. Q., Shi E. X., Zhang W. L, Yang B. S ., Electroanalysis, 2017,29(5), 1232— 1242 |
| [7] | Wang W.M., Zhao Y. Q., Wang H. F., Yang B. S ., Protein Science, 2018,27(6), 1102— 1108 |
| [8] | Duan L., Zhao Y.Q., Wang Z. J., Li G. T., Liang A. H., Yang B. S ., Journal of Inorganic Biochemistry, 2008,102(2), 268— 277 |
| [9] | Zhao Y.Q., Song L., Liang A. H., Yang B. S., . Journal of Photochemistry & Photobiology B: Biology, 2009,95(1), 26— 32 |
| [10] | Zhang W.L., Shi E. X., Zhao Y. Q., Yang B. S., . Journal of Inorganic Biochemistry, 2018,180, 15— 25 |
| [11] | Zhang W.L., Shi E. X., Feng Y. A., Yang B. S ., RSC Advances, 2017,7(82), 51773— 51788 |
| [12] | Laver D.R., Cherry C. A., Walker N. A., . Journal of Membrane Biology, 1997,155(3), 263— 274 |
| [13] | Barron E., Marshall R. J., Martorana M., Winslow E ., British Journal of Pharmacology, 2012,89(3), 603— 612 |
| [14] | Tanokura M., Yamada K ., Journal of Biological Chemistry, 1985,260(15), 8680— 8682 |
| [15] | Cinar S., Czeslik C ., Biochimica et Biophysica Acta(BBA)-Proteins and Proteomics, 2018,1866(5/6), 617— 623 |
| [16] | Feldkamp M.D., O’Donnell S. E., Yu L., Shea M. A ,. Proteins Structure Function and Bioinformatics, 2010,78(10), 2265— 2282 |
| [17] | Klee C.B., Crouch T. H., Richman P. G ., Advances in Protein Chemistry, 1982,35(1), 213— 321 |
| [18] | Zhao Y.Q., Yan J., Song L., Feng Y. N., Liang A. H., Yang B. S ., Spectrochimica Acta Part A: Molecular & Biomolecular Spectroscopy, 2012,87(4), 163— 170 |
| [19] | Song Z., Wang J. L., Yang B. S ., Spectrochimica Acta Part A: Molecular & Biomolecular Spectroscopy, 2014,118(2), 454— 460 |
| [20] | Ross P. D., Subramanian S ., Biochemistry, 1981,20(11), 3096— 3102 |
| [21] | Greenfield N. J ., Nature Protocols, 2006,1(6), 2876— 2890 |
| [22] | Ishtikhar M., Khan S., Badr G., Osama Mohamed A., Khan R. H ., Molecular Biosystems, 2014,10(11), 2954— 2964 |
| [23] | Li M., Zhang W. L., Yang B. S., . Journal of Inorganic Biochemistry, 2019,193, 15— 24 |
| [24] | Yang A., Miron S., Duchambon P., Assairi L., Blouquit Y., Craescu C. T ., Biochemistry, 2006,45(3), 880— 889 |
| [25] | Duan L., Liu W., Wang Z. J., Liang A. H., Yang B. S ., Journal of Biological Inorganic Chemistry, 2010,15(7), 995— 1007 |
| [26] | Wang Z. J., Zhao Y. Q., Ren L. X., Li G. T., Liang A. H., Yang B. S., . Journal of Photochemistry and Photobiology B: Biology, 2007,186(2/3), 178— 186 |
| [1] | CUI Shengfeng, WAN Jingwei, ZHOU Chenghe. Fluorescence Spectroscopic Analysis on the Synergistic Mechanism of Diazepam and Ethanol† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1178. |
| [2] | YANG Xin, ZHANG Di, SONG Limin, XU Qian, XU Hui, LIU Ke. Spectroscopic Studies on Alkaline Autoxidation of Curcumin and Antioxidative Activities of the Product† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1549. |
| [3] | LI Qing-Bo, SUN Xue-Jun, ZHANG Yuan-Fu, XU Yi-Zhuang, YANG Li-Min, SHI Jing-Sen, WU Jin-Guang . New Detection Method of Gastric Endoscope Samples Using Fourier Transform Mid-spectroscopy [J]. Chem. J. Chinese Universities, 2004, 25(9): 1624. |
| [4] | XU Xiao-Hua, YAO Guang-Min, LU Jian-Hua, LI Yan-Ming, LIN Chang-Jiang. A Novel Cerebroside from the Marine Sponge Phacellia fusca Schmidt [J]. Chem. J. Chinese Universities, 2001, 22(S1): 116. |
| [5] | FAN Ying-Fang, YANG Pin . Coordination Field Analysis of the Polarized Luminescence Spectra of Tb3+ in Trigonal Na3[Tb(C4H4O5)3]·2NaClO4·6H2O [J]. Chem. J. Chinese Universities, 1999, 20(8): 1266. |
| [6] | XU Xiao-Hua, SU Jing-Yu, ZENG Long-Mei, WANG Ming-Yan. Studies on the Chemical Constituent of the Alga Caloglossa Leprieurii [J]. Chem. J. Chinese Universities, 1998, 19(2): 249. |
| [7] | LI Yi-Mu, DU Zhao-Hui, DUAN Yi-Xiang, ZHANG Han-Qi, JIN Qin-Han, LIU Hong-Shi. The Research on the Spectral Analysis Characteristics of a Novel MPEGD Surce [J]. Chem. J. Chinese Universities, 1996, 17(2): 215. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||