

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (5): 872.doi: 10.7503/cjcu20141095
• Analytical Chemistry • Previous Articles Next Articles
CHENG Biaoping, LI Laisheng*( ), ZHOU Rendan, LI Liang, ZHANG Hongfu
), ZHOU Rendan, LI Liang, ZHANG Hongfu
Received:2014-12-15
Online:2015-05-10
Published:2015-04-15
Contact:
LI Laisheng
E-mail:lilaishengcn@163.com
Supported by:CLC Number:
TrendMD:
CHENG Biaoping, LI Laisheng, ZHOU Rendan, LI Liang, ZHANG Hongfu. Enantioseparations of Triazole Chiral Pesticides on Two β-Cyclodextrin-bonded Stationary Phases with Different Linkages by HPLC†[J]. Chem. J. Chinese Universities, 2015, 36(5): 872.
| CSP phase | EA(%) | Surface loadinga/(μmol·m-2) | TGA, w(%) | Surface loadingb/(μmol·m-2) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| CDSP | 3.61 | 0.89 | 0.33 | 0.156 | 8.2 | 0.149 |
| NCDSP | 3.17 | 1.03 | 0.30 | 0.137 | 7.1 | 0.129 |
Table 1 Elemental analysis(EA) and TGA results of CDSP and NCDSP
| CSP phase | EA(%) | Surface loadinga/(μmol·m-2) | TGA, w(%) | Surface loadingb/(μmol·m-2) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| CDSP | 3.61 | 0.89 | 0.33 | 0.156 | 8.2 | 0.149 |
| NCDSP | 3.17 | 1.03 | 0.30 | 0.137 | 7.1 | 0.129 |
| Pesticide | k'1 | k'2 | α1 | Rs1 | k'3 | k'4 | α2 | Rs2 | Mobile phase(volume ratio) | Column | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Triticonazole | 9.06 | 11.66 | 1.29 | 3.84 | H2O/ACN(75/25) | CDSP | |||||
| 10.58 | 10.79 | 1.02 | 0.66 | H2O/ACN(82.5/17.5) | NCDSP | ||||||
| Diniconazole | 11.23 | 14.42 | 1.28 | 3.23 | H2O/ACN(77.5/22.5) | CDSP | |||||
| 5.76 | 6.37 | 1.11 | 1.50 | H2O/ACN(80/20) | NCDSP | ||||||
| 2.75 | H2O/MeOH(20/80) | ADMPC* | [ | ||||||||
| 1.31 | H2O/ACN(50/50) | CDMPC* | [ | ||||||||
| Tebuconazole | 4.69 | 5.72 | 1.22 | 2.66 | H2O/ACN(72.5/27.5) | CDSP | |||||
| 5.59 | 5.98 | 1.07 | 1.35 | H2O/ACN(80/20) | NCDSP | ||||||
| 1.30 | H2O/ACN(50/50) | ADMPC | [ | ||||||||
| 0 | H2O/ACN(50/50) | CDMPC | [ | ||||||||
| Hexaconazole | 7.85 | 8.77 | 1.12 | 1.77 | H2O/ACN(80/20) | CDSP | |||||
| 9.17 | 10.1 | 1.17 | 2.42 | H2O/ACN(85/15) | NCDSP | ||||||
| 1.28 | H2O/ACN(55/45) | ADMPC | [ | ||||||||
| 1.72 | H2O/ACN(50/50) | CDMPC | [ | ||||||||
| Flutriafol | 3.42 | 3.96 | 1.16 | 1.74 | H2O/MeOH(65/35) | CDSP | |||||
| 6.91 | 7.87 | 1.14 | 1.79 | H2O/MeOH(80/20) | NCDSP | ||||||
| 1.05 | H2O/MeOH(30/70) | ADMPC | [ | ||||||||
| 0 | H2O/MeOH(30/70) | CDMPC | [ | ||||||||
| Uniconazole | 14.35 | 15.54 | 1.08 | 1.19 | H2O/ACN(80/20) | CDSP | |||||
| 8.19 | 8.59 | 1.05 | 0.69 | H2O/ACN(82.5/17.5) | NCDSP | ||||||
| 2.41 | H2O/ACN(40/60) | ADMPC | [ | ||||||||
| 0 | H2O/ACN(40/60) | CDMPC | [ | ||||||||
| Imazalil | 6.00 | 6.40 | 1.07 | 0.84 | 0.1%TEAA/ACN | CDSP | |||||
| (70/30), pH=5.0 | |||||||||||
| 6.74 | 7.38 | 1.09 | 1.30 | 0.1%TEAA/ACN | NCDSP | ||||||
| (82.5/17.5), pH=5.0 | |||||||||||
| Myclobutanil | 7.85 | 8.77 | 1.03 | <0.5 | H2O/ACN(85/15) | CDSP | |||||
| 8.34 | 8.34 | 1.00 | 0 | H2O/ACN(90/10) | NCDSP | ||||||
| Triadimenol | 5.27 | 5.27 | 1.00 | 0 | 10.33 | 11.2 | 1.08 | 1.01 | H2O/ACN(75/25) | CDSP | |
| 4.82 | 5.23 | 1.09 | 1.35 | 7.96 | 8.30 | 1.04 | <0.5 | H2O/ACN(82.5/17.5) | NCDSP | ||
| Triadimefon | 10.00 | 10.00 | 1.00 | 0 | H2O/ACN(80/20) | CDSP | |||||
| 8.00 | 8.00 | 1.00 | 0 | H2O/ACN(85/15) | NCDSP | ||||||
| 0 | H2O/MeOH(20/80) | ADMPC | [ | ||||||||
| 1.35 | H2O/MeOH(20/80) | CDMPC | [ |
Table 2 Separation results for triazole chiral pesticides on CDSP and NCDSP
| Pesticide | k'1 | k'2 | α1 | Rs1 | k'3 | k'4 | α2 | Rs2 | Mobile phase(volume ratio) | Column | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Triticonazole | 9.06 | 11.66 | 1.29 | 3.84 | H2O/ACN(75/25) | CDSP | |||||
| 10.58 | 10.79 | 1.02 | 0.66 | H2O/ACN(82.5/17.5) | NCDSP | ||||||
| Diniconazole | 11.23 | 14.42 | 1.28 | 3.23 | H2O/ACN(77.5/22.5) | CDSP | |||||
| 5.76 | 6.37 | 1.11 | 1.50 | H2O/ACN(80/20) | NCDSP | ||||||
| 2.75 | H2O/MeOH(20/80) | ADMPC* | [ | ||||||||
| 1.31 | H2O/ACN(50/50) | CDMPC* | [ | ||||||||
| Tebuconazole | 4.69 | 5.72 | 1.22 | 2.66 | H2O/ACN(72.5/27.5) | CDSP | |||||
| 5.59 | 5.98 | 1.07 | 1.35 | H2O/ACN(80/20) | NCDSP | ||||||
| 1.30 | H2O/ACN(50/50) | ADMPC | [ | ||||||||
| 0 | H2O/ACN(50/50) | CDMPC | [ | ||||||||
| Hexaconazole | 7.85 | 8.77 | 1.12 | 1.77 | H2O/ACN(80/20) | CDSP | |||||
| 9.17 | 10.1 | 1.17 | 2.42 | H2O/ACN(85/15) | NCDSP | ||||||
| 1.28 | H2O/ACN(55/45) | ADMPC | [ | ||||||||
| 1.72 | H2O/ACN(50/50) | CDMPC | [ | ||||||||
| Flutriafol | 3.42 | 3.96 | 1.16 | 1.74 | H2O/MeOH(65/35) | CDSP | |||||
| 6.91 | 7.87 | 1.14 | 1.79 | H2O/MeOH(80/20) | NCDSP | ||||||
| 1.05 | H2O/MeOH(30/70) | ADMPC | [ | ||||||||
| 0 | H2O/MeOH(30/70) | CDMPC | [ | ||||||||
| Uniconazole | 14.35 | 15.54 | 1.08 | 1.19 | H2O/ACN(80/20) | CDSP | |||||
| 8.19 | 8.59 | 1.05 | 0.69 | H2O/ACN(82.5/17.5) | NCDSP | ||||||
| 2.41 | H2O/ACN(40/60) | ADMPC | [ | ||||||||
| 0 | H2O/ACN(40/60) | CDMPC | [ | ||||||||
| Imazalil | 6.00 | 6.40 | 1.07 | 0.84 | 0.1%TEAA/ACN | CDSP | |||||
| (70/30), pH=5.0 | |||||||||||
| 6.74 | 7.38 | 1.09 | 1.30 | 0.1%TEAA/ACN | NCDSP | ||||||
| (82.5/17.5), pH=5.0 | |||||||||||
| Myclobutanil | 7.85 | 8.77 | 1.03 | <0.5 | H2O/ACN(85/15) | CDSP | |||||
| 8.34 | 8.34 | 1.00 | 0 | H2O/ACN(90/10) | NCDSP | ||||||
| Triadimenol | 5.27 | 5.27 | 1.00 | 0 | 10.33 | 11.2 | 1.08 | 1.01 | H2O/ACN(75/25) | CDSP | |
| 4.82 | 5.23 | 1.09 | 1.35 | 7.96 | 8.30 | 1.04 | <0.5 | H2O/ACN(82.5/17.5) | NCDSP | ||
| Triadimefon | 10.00 | 10.00 | 1.00 | 0 | H2O/ACN(80/20) | CDSP | |||||
| 8.00 | 8.00 | 1.00 | 0 | H2O/ACN(85/15) | NCDSP | ||||||
| 0 | H2O/MeOH(20/80) | ADMPC | [ | ||||||||
| 1.35 | H2O/MeOH(20/80) | CDMPC | [ |
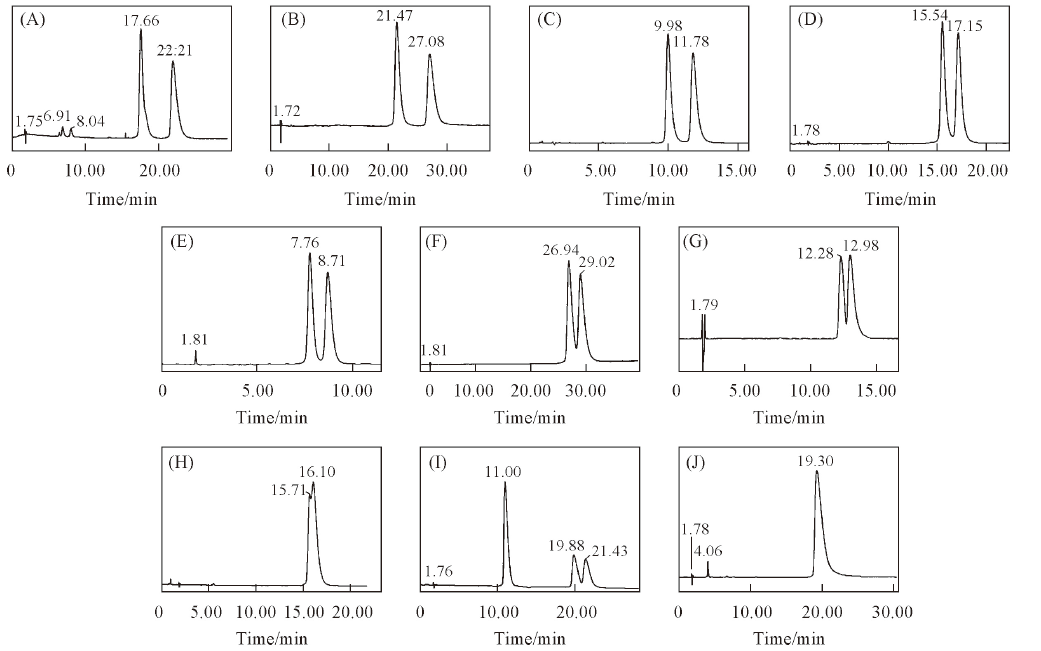
Fig.4 Chromatograms of 10 triazole chiral pesticides enantiomers on CDSP(A) Triticonazole; (B) diniconazole; (C) tebuconazole; (D) hexaconazole; (E) flutriafol; (F) uniconazole; (G) imazalil; (H) myclobutanil; (I) triadimenol; (J) triadimefon. Mobile phases: H2O or 0.1% TEAA-ACN or MeOH(Table 2); flow rate: 1.0 mL/min; UV: 220 nm.
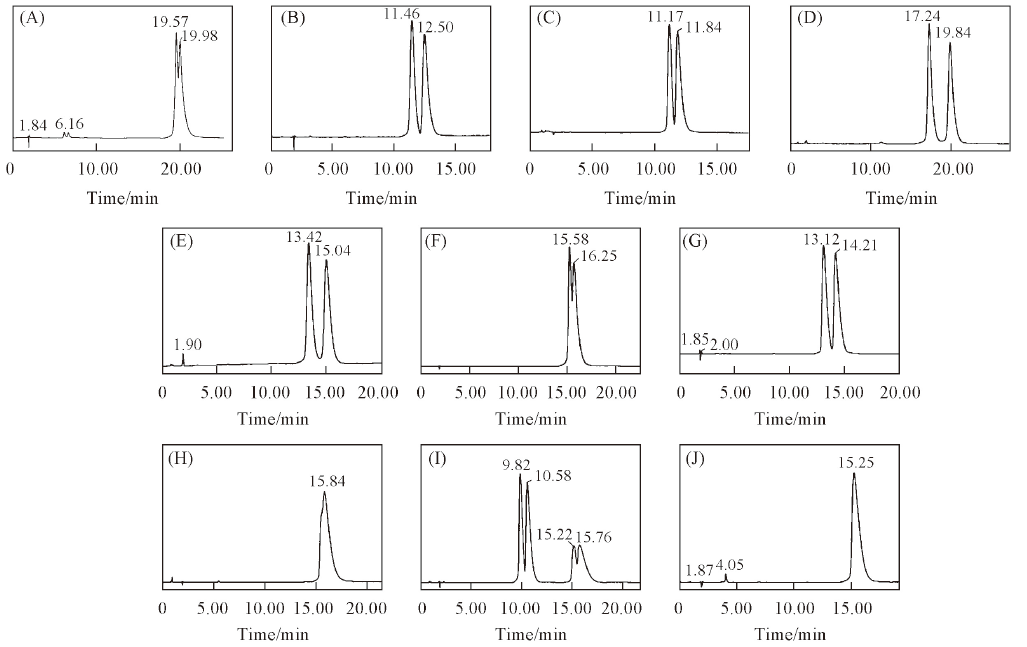
Fig.5 Chromatograms of 10 triazole chiral pesticides enantiomers on NCDSP(A) Triticonazole; (B) diniconazole; (C) tebuconazole; (D) hexaconazole; (E) flutriafol; (F) uniconazole; (G) imazalil; (H) myclobutanil; (I) triadimenol; (J) triadimefon. Mobile phases: H2O or 0.1% TEAA-ACN or MeOH(Table 2); flow rate: 1.0 mL/min; UV: 220 nm.
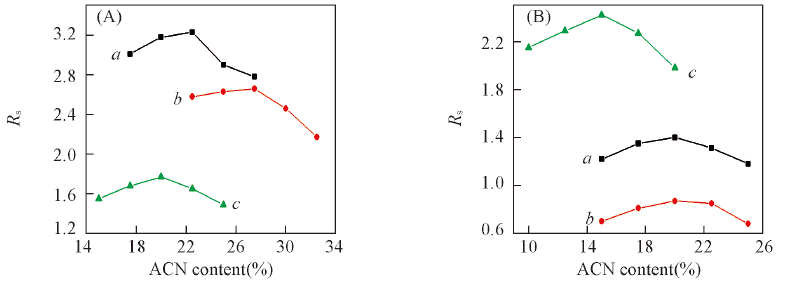
Fig.6 Effect of ACN content in the mobile phase on the resolution(Rs) of triazole pesticides on CDSP(A) and NCDSP(B) a. Diniconazol; b. tebuaconazole; c. hexaconazole. Mobile phase: ACN/H2O, flow rate: 1.0 mL/min.
| Column | T/K | 1/T | k1' | k2' | α | lnα | Rs | Mobile phase(volume ratio) |
|---|---|---|---|---|---|---|---|---|
| CDSP | 293 | 0.003413 | 7.85 | 8.77 | 1.12 | 0.1133 | 1.77 | H2O/ACN(80/20) |
| 298 | 0.003356 | 6.20 | 6.85 | 1.10 | 0.0953 | 1.57 | ||
| 303 | 0.003300 | 5.27 | 5.75 | 1.09 | 0.0862 | 1.42 | ||
| 308 | 0.003247 | 4.46 | 4.81 | 1.08 | 0.0770 | 1.23 | ||
| 313 | 0.003195 | 3.79 | 4.05 | 1.07 | 0.0677 | 1.08 | ||
| NCDSP | 293 | 0.003413 | 9.17 | 10.71 | 1.17 | 0.1570 | 2.42 | H2O/ACN(85/15) |
| 298 | 0.003356 | 6.72 | 7.68 | 1.14 | 0.1310 | 2.02 | ||
| 303 | 0.003300 | 5.70 | 6.41 | 1.12 | 0.1133 | 1.82 | ||
| 308 | 0.003247 | 4.74 | 5.27 | 1.11 | 0.1044 | 1.63 | ||
| 313 | 0.003195 | 3.99 | 4.37 | 1.10 | 0.0953 | 1.42 |
Table 3 Effect of temperature on the chiral separation of hexaconazole
| Column | T/K | 1/T | k1' | k2' | α | lnα | Rs | Mobile phase(volume ratio) |
|---|---|---|---|---|---|---|---|---|
| CDSP | 293 | 0.003413 | 7.85 | 8.77 | 1.12 | 0.1133 | 1.77 | H2O/ACN(80/20) |
| 298 | 0.003356 | 6.20 | 6.85 | 1.10 | 0.0953 | 1.57 | ||
| 303 | 0.003300 | 5.27 | 5.75 | 1.09 | 0.0862 | 1.42 | ||
| 308 | 0.003247 | 4.46 | 4.81 | 1.08 | 0.0770 | 1.23 | ||
| 313 | 0.003195 | 3.79 | 4.05 | 1.07 | 0.0677 | 1.08 | ||
| NCDSP | 293 | 0.003413 | 9.17 | 10.71 | 1.17 | 0.1570 | 2.42 | H2O/ACN(85/15) |
| 298 | 0.003356 | 6.72 | 7.68 | 1.14 | 0.1310 | 2.02 | ||
| 303 | 0.003300 | 5.70 | 6.41 | 1.12 | 0.1133 | 1.82 | ||
| 308 | 0.003247 | 4.74 | 5.27 | 1.11 | 0.1044 | 1.63 | ||
| 313 | 0.003195 | 3.99 | 4.37 | 1.10 | 0.0953 | 1.42 |
| [1] | Liu W. P., Gan J. Y., Schlenk D. P., Natl. Acad. Sci. USA,2005, 102(3), 701—706 |
| [2] | Crowell S. R., Henderson W. M., Kenneke J. F., Fisher J. W., Toxicol. Lett., 2011, 205(2), 154—162 |
| [3] | Goetz A. K., Dix D. J., Toxicol. Sci., 2009, 110(2), 449—462 |
| [4] | Toribio L., Bernal J. L., Martin M. T., Bernal J., del Nozal M. J., Biomed. Chromatogr., 2014, 28(1), 152—158 |
| [5] | Zhang C., Cai J. R., Duan Y. Q., Xu L., Fang G. Z., Wang S., Chem. Res. Chinese Universities,2014, 30(3), 374—378 |
| [6] | Otsuka K., Matsumura M., Kim J. B., Terabe S., J. Pharmaceut. Biomed., 2003, 30(6), 1861—1867 |
| [7] | Yang L., Liao Y., Zhou Z. Q., Jiang S. R., Wang P., J. Instr. Anal., 2004, 23(5), 133—135 |
| (杨丽, 廖勇, 周志强, 江树人, 王鹏. 分析测试学报, 2004, 23(5), 133—135) | |
| [8] | Pan C. X., Shen B. C., Xu B. J., Chen J. J., Xu X. Z., J. Sep. Sci., 2006, 29(13), 2004—2011 |
| [9] | Li Z. Y., Zhang Z.C., Zhou Q. L., J. Aoac Int., 2003, 86(3), 521—528 |
| [10] | Wang P., Jiang S. R., Liu D.H.,Wang P., Zhou Z. Q., J. Biochem. Biophy. Methods,2005, 62(3), 219—230 |
| [11] | Li C. Y., Zhang Y. C., Li Q. L., Wang W. X., Li J. Y., Chinese J. Anal. Chem., 2010, 38(2), 37—40 |
| (李朝阳, 张艳川, 李巧玲, 王未肖, 李景印. 分析化学, 2010, 38(2), 37—40) | |
| [12] | Zhang Q., Tian M. M., Wang M. Y., Shi H. Y., Wang M. H., J. Agr. Food. Chem., 2014, 62(13), 2809—2815 |
| [13] | Xiao Y., Ng S. C., Tan T. T. Y., Wang Y., J. Chromatogr. A,2012, 1269, 52—68 |
| [14] | Armstrong D. W., Chen S., Chang C., Chang S., J. Liq. Chromatogr. Rel. Technol., 1992, 15(3), 545—556 |
| [15] | Zhao D. Y., Huo Q. S., Feng J. L., Chmelka B. F., Stucky G. D., J. Am. Chem. Soc., 1998, 120(24), 6024—6036 |
| [16] | Zhou R. D., Li L. S., Cheng B. P., Nie G. Z., Zhang H. F., Acta Chim. Sinica,2014, 72(6), 720—730 |
| (周仁丹, 李来生, 程彪平, 聂桂珍, 张宏福. 化学学报, 2014, 72(6), 720—730) | |
| [17] | Zhou R. D., Li L. S., Cheng B. P., Nie G. Z., Zhang H. F., Chem. J. Chinese Universities,2014, 35(6), 1152—1160 |
| (周仁丹, 李来生, 程彪平, 聂桂珍, 张宏福. 高等学校化学学报, 2014, 35(6), 1152—1160) | |
| [18] | Zhong Q. Q., He L. F., Beesley T., Trahanovsky W. S., Sun P., Wang C. L., Armstrong D. W., J. Chromatogr. A,2006, 1115(1/2), 19—45 |
| [19] | Peter A., Torok G., Armstrong A. W., Toth G., Tourwe D., J. Chromatogr. A,1998, 828(1/2), 177—190 |
| [20] | Tian Q., Ren L. P., Lü C. G., Zhou Z. Q., Chinese J. Anal. Chem., 2010, 5(38), 688—692 |
| (田芹, 任丽萍, 吕春光, 周志强, 分析化学, 2010, 5(38), 688—692) |
| [1] | WANG Mingfang, FU Hua, FU Zhibo, WANG Yuerong, ZHANG Hongyang, ZHANG Min, HU Ping. Separation and Characterization of Polymer Blends Using Online Ultra-high Performance Liquid Chromatography-Size Exclusion Chromatography [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210865. |
| [2] | LI Jing, SU Wei, WANG Xueyuan, FU Peng, SUN Yan. Synthesis and Characterization of Antihypertensive Drug Aranidipine and Its Related Impurities [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210663. |
| [3] | WANG Tianqi,YU Qiongwei,FENG Yuqi. Analysis of Imidazole Propionic Acid in Serum of Patients with Type 2 Diabetes Based on NiO@SiO2 Solid-phase Extraction Coupled with Liquid Chromatography-Mass Spectrometry † [J]. Chem. J. Chinese Universities, 2020, 41(2): 262. |
| [4] | ZHANG Hui, ZHANG Chenjie, XU Minmin, YUAN Yaxian, YAO Jianlin. Investigation on the Reaction of o-Aminothiophenol and 2-Iodobenzoyl Chloride Monitored by SERS-HPLC Technique [J]. Chem. J. Chinese Universities, 2020, 41(11): 2496. |
| [5] | ZHAO Mengxin, MENG Zhe, LI Heping, MA Zongqin, ZHAN Haijuan, LIU Wanyi. Photodegradation of Antibiotic in Environmental Water by Graphene Oxide Modulation Bismuth Molybdate Under Visible Light Irradiation [J]. Chem. J. Chinese Universities, 2020, 41(11): 2479. |
| [6] | ZHAO Huanxi,WANG Qiuying,SUN Xiuli,LI Xue,MIAO Rui,WU Dongxue,LIU Shuying,XIU Yang. Discrimination of Ginseng Origins and Identification of Ginsenoside Markers Based on HPLC-MS Combined with Multivariate Statistical Analysis† [J]. Chem. J. Chinese Universities, 2019, 40(2): 246. |
| [7] | HE Yangyang,LI Yi,LI Baozong,YANG Yonggang. Biomimetic Mineralization at a Dilute Concentration and Application in Enantioseparation† [J]. Chem. J. Chinese Universities, 2019, 40(2): 393. |
| [8] | HUANG Weiwei, REN Jiawang, FANG Qianrong, Valentin VALTCHEV. Synthesis of Zeolite β@IISERP-COF2 Core-shell Hybrid Materials† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1127. |
| [9] | ZHOU Min, XU Xiaoying, LONG Yuande. Enantioseparation of Seventeen Kinds of β-Lactams on Carboxymethyl-β-cyclodextrin Chiral Stationary Phase and Research on Enantioseparation Mechanism [J]. Chem. J. Chinese Universities, 2018, 39(6): 1164. |
| [10] | SHI Penghui,BIAN Liujiao. Mechanism Study on the Interaction Between Cefoxitin and Metal β-Lactamase BcⅡ Based on Spectroscopic Methods and Computional Simulations† [J]. Chem. J. Chinese Universities, 2018, 39(5): 971. |
| [11] | QIU Xiuzhen, HUA Yongbiao, GUO Huishi, LU Wenguan. Preparation of a Molecularly Imprinted Polymer Nanotubes Membrane and Its Application in the Determination of Catecholamines in Urine Samples† [J]. Chem. J. Chinese Universities, 2018, 39(4): 653. |
| [12] | LIU Li, MA Yangyang, WANG Kuan, JIA Yunjing, LI Wan, ZHU Huajie. Anti-tumor and Antimicrobial Activities of β-Carbolines† [J]. Chem. J. Chinese Universities, 2018, 39(4): 674. |
| [13] | YANG Qinghua, WANG Longgang, LIU Jie, LU Yong, CHEN Tianyun. Preparation and Characterization of Star-shaped β-Cyclodextrin Based Polymer† [J]. Chem. J. Chinese Universities, 2018, 39(4): 793. |
| [14] | ZHANG Jin, SHI Tiancai, LUO Liwen, LIU Jia, LIU Rong, LIU Le, LIANG Ming, MA Yangmin. One-pot Synthesis of β-Carboline Derivatives Catalyzed by CuO Nanoparticles† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2411. |
| [15] | LIU Xinru, LIU Chunying, XU Longquan, SONG Jianguo, YU Hongshan. Construction of Ginsenoside-β-glucosidase Gene Vector and Biotransformation in Pichia Pastoris† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2451. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||