

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (1): 121.doi: 10.7503/cjcu20130448
• Physical Chemistry • Previous Articles Next Articles
HOU Haiyun1,2,*( ), HUANG Yinrong1, BAI Bofeng2, YANG Jing1
), HUANG Yinrong1, BAI Bofeng2, YANG Jing1
Received:2013-05-13
Online:2014-01-10
Published:2013-06-13
Contact:
HOU Haiyun
E-mail:houhaiyun77@126.com
Supported by:CLC Number:
TrendMD:
HOU Haiyun, HUANG Yinrong, BAI Bofeng, YANG Jing. Volumetric Properties and Molecular Interactions of Binary Mixtures Imidazolium Acetates-ethanol at 293.15 K†[J]. Chem. J. Chinese Universities, 2014, 35(1): 121.
| Binary mixture | x1 | x2 | ρ/(g·cm-3) | φV1/(cm3·mol-1) | φV2/(cm3·mol-1) | |
|---|---|---|---|---|---|---|
| [Mim]Ac(1)-EtOH(2) | 0.0000 | 1.0000 | 0.7895 | 58.27 | 0.000 | |
| 0.0275 | 0.9725 | 0.8123 | 117.13 | 58.10 | -0.162 | |
| 0.0352 | 0.9648 | 0.8184 | 117.38 | 58.06 | -0.198 | |
| 0.0478 | 0.9522 | 0.8280 | 117.76 | 58.00 | -0.251 | |
| 0.0629 | 0.9371 | 0.8393 | 117.95 | 57.92 | -0.319 | |
| 0.1514 | 0.8486 | 0.8981 | 118.85 | 57.52 | -0.631 | |
| 0.1709 | 0.8291 | 0.9098 | 118.93 | 57.42 | -0.699 | |
| 0.2135 | 0.7865 | 0.9334 | 119.28 | 57.25 | -0.798 | |
| 0.2603 | 0.7397 | 0.9571 | 119.59 | 57.06 | -0.892 | |
| 0.3396 | 0.6604 | 0.9927 | 120.08 | 56.76 | -0.997 | |
| 0.5022 | 0.4978 | 1.0505 | 121.03 | 56.26 | -0.997 | |
| 0.6015 | 0.3985 | 1.0787 | 121.50 | 55.98 | -0.912 | |
| 0.7299 | 0.2701 | 1.1087 | 122.06 | 55.69 | -0.696 | |
| 0.9025 | 0.0975 | 1.1408 | 122.73 | 55.57 | -0.263 | |
| 0.9384 | 0.0616 | 1.1467 | 122.84 | 55.49 | -0.171 | |
| 1.0000 | 0.0000 | 1.1561 | 123.02 | 0.000 | ||
| [Mmim]Ac(1)-EtOH(2) | 0.0000 | 1.0000 | 0.7895 | 58.27 | 0.000 | |
| 0.0291 | 0.9709 | 0.8147 | 131.67 | 58.06 | -0.195 | |
| 0.0405 | 0.9595 | 0.8236 | 132.57 | 58.02 | -0.236 | |
| 0.0513 | 0.9487 | 0.8321 | 132.65 | 57.96 | -0.294 | |
| 0.0733 | 0.9267 | 0.8483 | 133.11 | 57.85 | -0.387 | |
| 0.1157 | 0.8843 | 0.8773 | 133.47 | 57.62 | -0.569 | |
| 0.2104 | 0.7896 | 0.9318 | 134.26 | 57.17 | -0.867 | |
| 0.2315 | 0.7685 | 0.9424 | 134.40 | 57.07 | -0.922 | |
| 0.2607 | 0.7393 | 0.9561 | 134.61 | 56.93 | -0.984 | |
| [Mmim]Ac(1)-EtOH(2) | 0.3185 | 0.6815 | 0.9805 | 135.05 | 56.71 | -1.062 |
| 0.4929 | 0.5071 | 1.0379 | 136.18 | 56.12 | -1.087 | |
| 0.5903 | 0.4097 | 1.0620 | 136.72 | 55.88 | -0.979 | |
| 0.7305 | 0.2695 | 1.0899 | 137.41 | 55.62 | -0.714 | |
| 0.8659 | 0.1341 | 1.1115 | 137.94 | 55.40 | -0.385 | |
| 0.9306 | 0.0694 | 1.1203 | 138.16 | 55.31 | -0.205 | |
| 1.0000 | 0.0000 | 1.1289 | 138.38 | 0.000 | ||
| [Emim]Ac(1)-EtOH(2) | 0.0000 | 1.0000 | 0.7895 | 58.27 | 0.000 | |
| 0.0506 | 0.9494 | 0.8320 | 148.70 | 57.96 | -0.287 | |
| 0.0617 | 0.9383 | 0.8402 | 149.07 | 57.92 | -0.327 | |
| 0.0701 | 0.9299 | 0.8466 | 148.88 | 57.85 | -0.384 | |
| 0.0837 | 0.9163 | 0.8562 | 149.11 | 57.79 | -0.440 | |
| 0.1103 | 0.8897 | 0.8741 | 149.28 | 57.64 | -0.561 | |
| 0.1919 | 0.8081 | 0.9207 | 149.92 | 57.21 | -0.854 | |
| 0.2246 | 0.7754 | 0.9365 | 150.18 | 57.05 | -0.939 | |
| 0.2375 | 0.7625 | 0.9424 | 150.29 | 57.00 | -0.968 | |
| 0.2691 | 0.7309 | 0.9559 | 150.52 | 56.85 | -1.035 | |
| 0.4627 | 0.5373 | 1.0189 | 151.83 | 56.08 | -1.172 | |
| 0.5714 | 0.4286 | 1.0433 | 152.51 | 55.80 | -1.058 | |
| 0.7035 | 0.2965 | 1.0668 | 153.18 | 55.45 | -0.834 | |
| 0.8771 | 0.1229 | 1.0899 | 153.93 | 55.14 | -0.384 | |
| 0.9267 | 0.0733 | 1.0954 | 154.11 | 55.06 | -0.235 | |
| 1.0000 | 0.0000 | 1.1027 | 154.37 | 0.000 |
Table 1 Molar fractions, densities, apparent molar volumes and excess molar volumes of the binary mixtures [Mim]Ac/[Mmim]Ac/[Emim]Ac(1)-EtOH(2) at 293.15 K
| Binary mixture | x1 | x2 | ρ/(g·cm-3) | φV1/(cm3·mol-1) | φV2/(cm3·mol-1) | |
|---|---|---|---|---|---|---|
| [Mim]Ac(1)-EtOH(2) | 0.0000 | 1.0000 | 0.7895 | 58.27 | 0.000 | |
| 0.0275 | 0.9725 | 0.8123 | 117.13 | 58.10 | -0.162 | |
| 0.0352 | 0.9648 | 0.8184 | 117.38 | 58.06 | -0.198 | |
| 0.0478 | 0.9522 | 0.8280 | 117.76 | 58.00 | -0.251 | |
| 0.0629 | 0.9371 | 0.8393 | 117.95 | 57.92 | -0.319 | |
| 0.1514 | 0.8486 | 0.8981 | 118.85 | 57.52 | -0.631 | |
| 0.1709 | 0.8291 | 0.9098 | 118.93 | 57.42 | -0.699 | |
| 0.2135 | 0.7865 | 0.9334 | 119.28 | 57.25 | -0.798 | |
| 0.2603 | 0.7397 | 0.9571 | 119.59 | 57.06 | -0.892 | |
| 0.3396 | 0.6604 | 0.9927 | 120.08 | 56.76 | -0.997 | |
| 0.5022 | 0.4978 | 1.0505 | 121.03 | 56.26 | -0.997 | |
| 0.6015 | 0.3985 | 1.0787 | 121.50 | 55.98 | -0.912 | |
| 0.7299 | 0.2701 | 1.1087 | 122.06 | 55.69 | -0.696 | |
| 0.9025 | 0.0975 | 1.1408 | 122.73 | 55.57 | -0.263 | |
| 0.9384 | 0.0616 | 1.1467 | 122.84 | 55.49 | -0.171 | |
| 1.0000 | 0.0000 | 1.1561 | 123.02 | 0.000 | ||
| [Mmim]Ac(1)-EtOH(2) | 0.0000 | 1.0000 | 0.7895 | 58.27 | 0.000 | |
| 0.0291 | 0.9709 | 0.8147 | 131.67 | 58.06 | -0.195 | |
| 0.0405 | 0.9595 | 0.8236 | 132.57 | 58.02 | -0.236 | |
| 0.0513 | 0.9487 | 0.8321 | 132.65 | 57.96 | -0.294 | |
| 0.0733 | 0.9267 | 0.8483 | 133.11 | 57.85 | -0.387 | |
| 0.1157 | 0.8843 | 0.8773 | 133.47 | 57.62 | -0.569 | |
| 0.2104 | 0.7896 | 0.9318 | 134.26 | 57.17 | -0.867 | |
| 0.2315 | 0.7685 | 0.9424 | 134.40 | 57.07 | -0.922 | |
| 0.2607 | 0.7393 | 0.9561 | 134.61 | 56.93 | -0.984 | |
| [Mmim]Ac(1)-EtOH(2) | 0.3185 | 0.6815 | 0.9805 | 135.05 | 56.71 | -1.062 |
| 0.4929 | 0.5071 | 1.0379 | 136.18 | 56.12 | -1.087 | |
| 0.5903 | 0.4097 | 1.0620 | 136.72 | 55.88 | -0.979 | |
| 0.7305 | 0.2695 | 1.0899 | 137.41 | 55.62 | -0.714 | |
| 0.8659 | 0.1341 | 1.1115 | 137.94 | 55.40 | -0.385 | |
| 0.9306 | 0.0694 | 1.1203 | 138.16 | 55.31 | -0.205 | |
| 1.0000 | 0.0000 | 1.1289 | 138.38 | 0.000 | ||
| [Emim]Ac(1)-EtOH(2) | 0.0000 | 1.0000 | 0.7895 | 58.27 | 0.000 | |
| 0.0506 | 0.9494 | 0.8320 | 148.70 | 57.96 | -0.287 | |
| 0.0617 | 0.9383 | 0.8402 | 149.07 | 57.92 | -0.327 | |
| 0.0701 | 0.9299 | 0.8466 | 148.88 | 57.85 | -0.384 | |
| 0.0837 | 0.9163 | 0.8562 | 149.11 | 57.79 | -0.440 | |
| 0.1103 | 0.8897 | 0.8741 | 149.28 | 57.64 | -0.561 | |
| 0.1919 | 0.8081 | 0.9207 | 149.92 | 57.21 | -0.854 | |
| 0.2246 | 0.7754 | 0.9365 | 150.18 | 57.05 | -0.939 | |
| 0.2375 | 0.7625 | 0.9424 | 150.29 | 57.00 | -0.968 | |
| 0.2691 | 0.7309 | 0.9559 | 150.52 | 56.85 | -1.035 | |
| 0.4627 | 0.5373 | 1.0189 | 151.83 | 56.08 | -1.172 | |
| 0.5714 | 0.4286 | 1.0433 | 152.51 | 55.80 | -1.058 | |
| 0.7035 | 0.2965 | 1.0668 | 153.18 | 55.45 | -0.834 | |
| 0.8771 | 0.1229 | 1.0899 | 153.93 | 55.14 | -0.384 | |
| 0.9267 | 0.0733 | 1.0954 | 154.11 | 55.06 | -0.235 | |
| 1.0000 | 0.0000 | 1.1027 | 154.37 | 0.000 |
| Binary mixture | φ | a/(cm3·mol-1) | b/(cm3·mol-1) | σ/(cm3·mol-1) | ||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (1) | (2) | (1) | (2) | (1) | (2) | |
| [Mim]Ac(1)-EtOH(2) | 117.05 | 55.54 | 10.12 | 0.27 | -4.31 | 2.54 | 0.07 | 0.02 |
| [Mmim]Ac(1)-EtOH(2) | 131.91 | 55.38 | 11.11 | 0.22 | -4.78 | 2.73 | 0.07 | 0.02 |
| [Emim]Ac(1)-EtOH(2) | 148.25 | 55.10 | 9.07 | 0.54 | -2.95 | 2.70 | 0.07 | 0.02 |
Table 2 Least-square fitting parameters φVB0, a, b and standard deviations σ of Eq. (4) for binary mixtures [Mim]Ac/[Mmim]Ac/[Emim]Ac(1)-EtOH(2)
| Binary mixture | φ | a/(cm3·mol-1) | b/(cm3·mol-1) | σ/(cm3·mol-1) | ||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (1) | (2) | (1) | (2) | (1) | (2) | |
| [Mim]Ac(1)-EtOH(2) | 117.05 | 55.54 | 10.12 | 0.27 | -4.31 | 2.54 | 0.07 | 0.02 |
| [Mmim]Ac(1)-EtOH(2) | 131.91 | 55.38 | 11.11 | 0.22 | -4.78 | 2.73 | 0.07 | 0.02 |
| [Emim]Ac(1)-EtOH(2) | 148.25 | 55.10 | 9.07 | 0.54 | -2.95 | 2.70 | 0.07 | 0.02 |
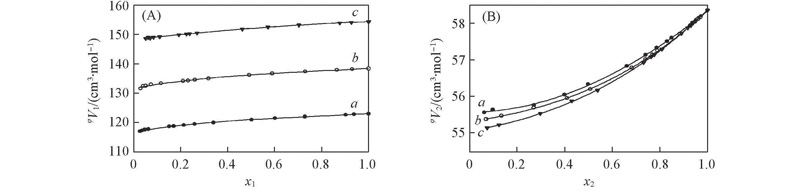
Fig.1 Fitting curves of the apparent molar volumes vs. molar fractions for binary mixtures[Mim]Ac/[Mmim]Ac/[Emim]Ac(1)-EtOH(2) at 293.15 K(A) φV1-x1; (B) φV2-x2. a. [Mim]Ac(1)-EtOH(2); b. [Mmim]Ac(1)-EtOH(2); c. [Emim]Ac(1)-EtOH(2).
| Binary mixture | δB/(cm3·mol-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| (1)a | (1)b | (2)a | (2)b | (1) | (2) | (1) | (2) | |
| [Mim]Ac(1)-EtOH(2) | 122.97 | 122.91 | 58.37 | 58.35 | 6.14 | 2.83 | 0.050 | 0.048 |
| [Mmim]Ac(1)-EtOH(2) | 138.36 | 138.29 | 58.37 | 58.33 | 6.45 | 2.99 | 0.047 | 0.051 |
| [Emim]Ac(1)-EtOH(2) | 154.37 | 154.37 | 58.37 | 58.34 | 6.12 | 3.27 | 0.040 | 0.056 |
Table 3 Molar volumes, the differences of the molar volumes and limiting partial molar volumes, solvation coefficients at infinite solutions of components (1) and (2) for [Mim]Ac/[Mmim]Ac/[Emim]Ac(1)-EtOH(2) at 293.15 K
| Binary mixture | δB/(cm3·mol-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| (1)a | (1)b | (2)a | (2)b | (1) | (2) | (1) | (2) | |
| [Mim]Ac(1)-EtOH(2) | 122.97 | 122.91 | 58.37 | 58.35 | 6.14 | 2.83 | 0.050 | 0.048 |
| [Mmim]Ac(1)-EtOH(2) | 138.36 | 138.29 | 58.37 | 58.33 | 6.45 | 2.99 | 0.047 | 0.051 |
| [Emim]Ac(1)-EtOH(2) | 154.37 | 154.37 | 58.37 | 58.34 | 6.12 | 3.27 | 0.040 | 0.056 |
| Binary mixture | A0 | A1 | A2 | A3 | σ/(cm3·mol-1) |
|---|---|---|---|---|---|
| [Mim]Ac(1)-EtOH(2) | -4.017 | -1.064 | -0.181 | -0.488 | 0.005 |
| [Mmim]Ac(1)-EtOH(2) | -4.309 | -1.484 | -0.218 | -0.118 | 0.006 |
| [Emim]Ac(1)-EtOH(2) | -4.572 | -1.524 | -0.078 | 0.224 | 0.005 |
Table 4 Redlich-Kister coefficients A0, A1, A2, A3 and standard deviation σ of Eq.(11) for binary mixtures [Mim]Ac/[Mmim]Ac/[Emim]Ac(1)-EtOH(2) at 293.15 K
| Binary mixture | A0 | A1 | A2 | A3 | σ/(cm3·mol-1) |
|---|---|---|---|---|---|
| [Mim]Ac(1)-EtOH(2) | -4.017 | -1.064 | -0.181 | -0.488 | 0.005 |
| [Mmim]Ac(1)-EtOH(2) | -4.309 | -1.484 | -0.218 | -0.118 | 0.006 |
| [Emim]Ac(1)-EtOH(2) | -4.572 | -1.524 | -0.078 | 0.224 | 0.005 |
| [1] | Wilkes J.S., Zaworotko M. J., J. Chem. Soc. Chem. Commun., 1992, (13), 965—967 |
| [2] | Welton T., Chem. Rev., 1999, 99,2071—2083 |
| [3] | Gao J., Luo Z. G., Luo F. X., Carbohydr. Polym., 2012, 89(4), 1215—1221 |
| [4] | Zhao H., Jackson L., Song Z. Y., Tetrahedron-Asymmetr., 2006, 17(17), 2491—2498 |
| [5] | Manohar C. V., Tamal B., Kaustubha M., J. Mol. Liq., 2013, 180, 145—153 |
| [6] | Udaya K. R., Tamal B., Fluid Phase Equilib., 2012, 324(25), 17—27 |
| [7] | Weerachanchai P., Leong S. S. J., Chang M. W., Ching C. B., Lee J. M., Bioresour. Technol., 2012, 111, 453—459 |
| [8] | Silva A. S., Lee S. H., Endo T., Bon E. P. S., Bioresour. Technol., 2011, 102(22), 10505—10509 |
| [9] | Handy S. T., Chem. Eur., 2003, 9, 2938—2944 |
| [10] | Liu Q. B., Zhang Z. H., Zhang F. J., Synthesisb and Application of a Nontoxic Ionic Liquids. CP 1854120A, 2006-11-01 |
| (刘庆彬, 张占辉, 张福军.无毒离子液体的制备方法及其应用,CP 1854120A, 2006-11-01) | |
| [11] | Won S. W., Choi S. B., Mao J., Yun Y. S., J. Hazard. Mater., 2013, 244/245, 130—134 |
| [12] | Choi S. B., Won S. W., Yun Y. S., Chem. Eng. J., 2013, 214, 78—82 |
| [13] | Zavrel M., Bross D., Funke M., Büchs J., Spiess A. C., Bioresour. Technol., 2009, 100, 2580—2587 |
| [14] | Liu H. B., Cheng G., Kent M., Stavila V., Simmons B. A., Sale K. L., Sing S., J. Phys. Chem. B, 2012, 116, 8131—8138 |
| [15] | Ding Z. D., Chi Z., Gu W. X., Gu S. M., Liu J. H., Wang H. J., Carbohydr. Polym., 2012, 89(1), 7—16 |
| [16] | Lynam J. G., Reza M. T., Vasquez V. R., Coronella C. J., Bioresour. Technol., 2012, 114, 629—636 |
| [17] | Zhang S.J., Xu C. M., Lü X. M., Zhou Q., Ionic Liquids and Green Chemistry, Science Press, Beijing, 2009, 606—612 |
| (张锁江, 徐春明, 吕兴梅, 周清. 离子液体与绿色化学, 北京: 科学出版社, 2009, 606—612) | |
| [18] | Gräsvik J., Eliasson B., Mikkola J. P., J. Mol. Struct., 2012, 1028, 156—163 |
| [19] | Michael F., Pascale C., Michael F. C., Carbohydr. Polym., 2012, 87(2), 1124—1130 |
| [20] | Ma X. X., Li L., Wei J., Duan W. B., Guan W., Yang J. Z., J. Chem. Eng. Data, 2012, 57(11), 3171—3175 |
| [21] | Guan W., Ma X. X., Li L., Tong J., Fang D. W., Yang J. Z., J. Phys. Chem. B, 2011, 115(44), 12915—12920 |
| [22] | Ding Z. D., Chi Z., Gu W. X., Gu S. M., Wang H. J., J. Mol. Struct., 2012, 1015, 147—155 |
| [23] | Hou H. Y., Huang Y. R., Wang S. Z., Bai B. F., Acta Phys. Chim. Sin., 2011, 27(11), 2512—2520 |
| (侯海云, 黄银蓉, 王升泽, 白博峰.物理化学学报, 2011,27(11), 2512—2520) | |
| [24] | Fudan Universityet al, Cai X. E., Xiang Y. F., Liu Y. G., Physical Chemistry Experiments & Techniques, 2nd Ed., Higher Education Press, Beijing, 1992, 446—448 |
| (复旦大学等编, 蔡显鄂, 项一非, 刘衍光修订. 物理化学实验, 第2版, 北京: 高等教育出版社, 1992, 446—448 | |
| [25] | Tasic D. R., Klofutar C., Monatshefte Für Chemie, 1998, 129(12), 1245—1257 |
| [26] | Michael Blandamer J., Chem. Soc. Rev., 1998, 27, 73—79 |
| [27] | Wawer J., Krakowiak J., Placzek A., Grzybkowski W., J. Mol. Liq., 2008, 143, 95—99 |
| [28] | Gong X. J., Guo Y. S., Yang Y. Z., Zhang L. L., Fang W. J., Lin R. S., Chem. J. Chinese Universities, 2012, 33(11), 2509—2515 |
| (龚先杰, 郭永胜, 杨玉忠, 张玲玲, 方文军, 林瑞森.高等学校化学学报, 2012,33(11), 2509—2515) | |
| [29] | Hou H. Y., Peng S. J., Wang S. Z., Geng X. P., Chem. Res. Chinese Universities, 2010, 26(2), 309—317 |
| [30] | Liu D. X., Li H. R., Deng D. S., Han S. J., Chinese J. Chem. Eng., 2002, 10(4), 454—458 |
| [31] | Perron G., Desnoyers J. E., J. Chem. Thermodyn., 1981, 13, 1105—1121 |
| [32] | Hou H. Y., Wang X. X., Peng S. J., Geng X. P., Chem. J. Chinese Universities, 2009, 30(7), 1386—1391 |
| (侯海云, 王晓先, 彭三军, 耿信鹏.高等学校化学学报, 2009,30(7), 1386—1391) | |
| [33] | Hou H. Y., Peng S. J., Wang X. X., Geng X. P., Chem. J. Chinese Universities, 2009, 30(3), 563—567 |
| (侯海云, 彭三军, 王晓先, 耿信鹏.高等学校化学学报, 2009,30(3), 563—567) | |
| [34] | Peng S. J., Hou H. Y., Zhou C. S., Yang T., J. Solution Chem., 2007, 36(8), 981—995 |
| [1] | XU Wenzhe, ZHANG Hao. Supramolecular Interactions-mediated Nanodrug Nucleation [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220264. |
| [2] | MA Zhuoyuan, WANG Dayang. Status and Prospect of Surface Wettability of Molecular Self-assembled Monolayers [J]. Chem. J. Chinese Universities, 2021, 42(4): 1031. |
| [3] | YU Yongbo,LIU Cui,GONG Lidong. Studies of (CH3OH)n(n=3—12) and [Na(CH3OH)n]+(n=3—6)via ab initio and ABEEMσπ/MM† [J]. Chem. J. Chinese Universities, 2016, 37(8): 1468. |
| [4] | SI Pengfei, LUO Faliang, HAI Mei. Intermolecular Interactions and Crystallization and Melting Behavior of Poly(L-lactic acid)/4,4'-Thiobis Phenol Blends† [J]. Chem. J. Chinese Universities, 2015, 36(1): 188. |
| [5] | YUAN Wei, REN Qingjiang, SUN Hengda, LI Hui, CHENG Yanxiang, MA Dongge. Effect of Peripheral Substituents on Luminescent Properties of the Porphyrin Platinum(Ⅱ) Complexes† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1229. |
| [6] | LI Dan, FANG Wen-Jun, LIU Li, WU Qian, ZHANG Hong-Yan, SUN Xiao-Ri. Volumetric and Viscous Properties for Binary Mixtures of N-Methylpiperazine with Ethyl Acetate or Butyl Acetate from 298.15 K to 313.15 K [J]. Chem. J. Chinese Universities, 2013, 34(8): 1924. |
| [7] | MENG Su-Ci, YIN Xiu-Lian, MA Jing, XIE Ji-Min. Theoretical Studies on Solvent Effects and Intermolecular Interactions of Organic π-Conjugated Ligand in Solutions [J]. Chem. J. Chinese Universities, 2012, 33(11): 2492. |
| [8] | LIANG Xue, SUN Tao, WANG Yi-Bo. Symmetry-adapted Perturbation Theory Study on the Nature of Benzene-halogen(X2, X=F, Cl, Br, I) [J]. Chem. J. Chinese Universities, 2012, 33(03): 541. |
| [9] | HOU Hai-Yun*, WANG Xiao-Xian, PENG San-Jun, GENG Xin-Peng. Volumetric Properties of Binary System C6H5CH3-DMF at 293.15 K [J]. Chem. J. Chinese Universities, 2009, 30(7): 1386. |
| [10] | HOU Hai-Yun1*, PENG San-Jun2, WANG Xiao-Xian1, GENG Xin-Peng1. Volumetric Properties of Binary System C6H6-DMF at 293.15 K [J]. Chem. J. Chinese Universities, 2009, 30(3): 563. |
| [11] | ZHUO Ke-Lei1*, LIU Yao-Hui1, ZHANG Qiu-Fen2, LIU Hong-Xun1, WANG Jian-Ji1. Volumetric Properties of the Interaction of Arabinose with HCl Aqueous Solution at Different Temperatures [J]. Chem. J. Chinese Universities, 2008, 29(5): 963. |
| [12] | ZHAO Jian-Xin1, QIAO Yi-Tao1, FENG Jing1, LUO Zhao-Feng2, YUAN Zhi1*. Interaction of Copolymer-Zn with Polypeptide [J]. Chem. J. Chinese Universities, 2008, 29(3): 658. |
| [13] |
YANG Bing, MA Yu-Guang*, SHEN Jia-Cong*.
Stacking Mode, Optoelectronic Property and Supramolecular Control Method in π\|Conjugated Organic Molecules [J]. Chem. J. Chinese Universities, 2008, 29(12): 2643. |
| [14] | LING Xiao-Mei1, LIU Yi1, LAI Xian-Yin2, ZHANG Yuan2, LIU Xiao-Ming2, TU Peng-Fei2, ZHAO Yu-Ying2,3, CUI Jing-Rong3. Application of Capillary Electrophoresis to ScreeningNatural Products Using Thrombin as Target [J]. Chem. J. Chinese Universities, 2007, 28(2): 234. |
| [15] | WANG Zhao-Xu, ZHANG Jing-Chang, CAO Wei-liang. Theoretical Study on Intermolecular Interactions BetweenHCN(HNC) and NH3, H2O, HF [J]. Chem. J. Chinese Universities, 2007, 28(2): 320. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||