

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (4): 811.doi: 10.7503/cjcu20190596
• Polymer Chemistry • Previous Articles Next Articles
ZUO Xiaoling1,*( ),WU Chong2,HUANG Anrong3,LUO Jiaolian1,4,LI Zhuyu1,WANG Meng1,ZHOU Ying1,YU Hongna1,GUO Jianbing1,*(
),WU Chong2,HUANG Anrong3,LUO Jiaolian1,4,LI Zhuyu1,WANG Meng1,ZHOU Ying1,YU Hongna1,GUO Jianbing1,*( )
)
Received:2019-11-18
Online:2020-04-10
Published:2020-02-10
Contact:
Xiaoling ZUO,Jianbing GUO
E-mail:shanghai0401@163.com;guojianbing_1015@126.com
Supported by:CLC Number:
TrendMD:
ZUO Xiaoling, WU Chong, HUANG Anrong, LUO Jiaolian, LI Zhuyu, WANG Meng, ZHOU Ying, YU Hongna, GUO Jianbing. Visible-light-sensitive Versatile Fluorescent Brightener-based Photoinitiating Systems †[J]. Chem. J. Chinese Universities, 2020, 41(4): 811.
| OBs | λmax/nm | εmax/(L·mol-1·cm-1) | ε420 nm/(L·mol-1·cm-1) |
|---|---|---|---|
| BBS | 376 | Poor solubility | Poor solubility |
| C 1 | 373 | 32530±430 | 333±12 |
| CBUS 450 | 361 | 39010±750 | 58±34 |
| CBS X | 357 | 75800±1020 | 80±13 |
| OB 7 | 374 | 34290±1380 | 570±38 |
| OBs | λmax/nm | εmax/(L·mol-1·cm-1) | ε420 nm/(L·mol-1·cm-1) |
|---|---|---|---|
| BBS | 376 | Poor solubility | Poor solubility |
| C 1 | 373 | 32530±430 | 333±12 |
| CBUS 450 | 361 | 39010±750 | 58±34 |
| CBS X | 357 | 75800±1020 | 80±13 |
| OB 7 | 374 | 34290±1380 | 570±38 |
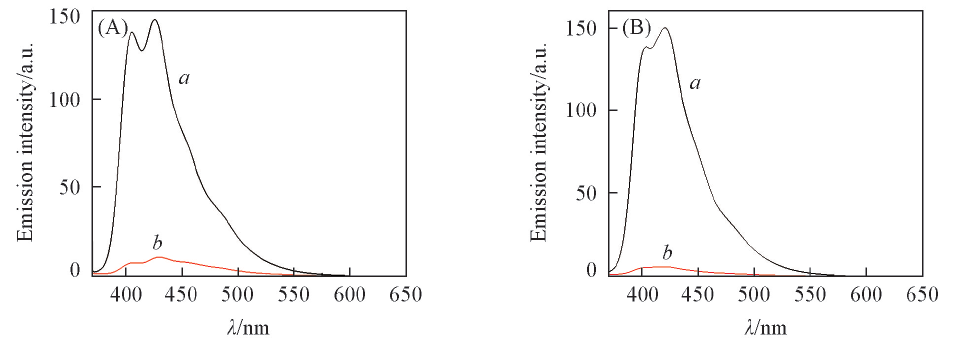
Fig.4 Fluorescence emission spectra of CBUS 450(A) and CBS X(B) in acetonitrile in the absence(a) and in the presence(b) of IOD (A) cIOD=1.0 mmol/L;(B) cIOD=4.3 mmol/L.
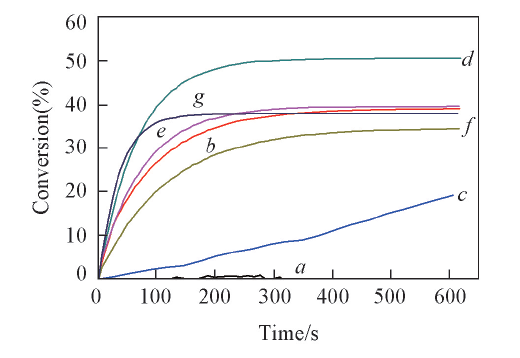
Fig.6 Photopolymerization profiles(acrylate function conversion vs time) of an Ebecryl 605/TMPTA(mass ratio, 70∶30) blends under air and in presence of BBS/IOD(a), OB 7/IOD(b), CBUS 450/IOD(c), CBS X/IOD(d), C 1/IOD(e), Ir369(1%, mass fraction, f) and Ir819(1%, mass fraction, g)
| PIS | FC(%) | PR | PIS | FC(%) | PR |
|---|---|---|---|---|---|
| OBs alone | np* | np | CBS X/IOD(0.2%/1%) | 50.7 | 0.71 |
| IOD alone | np | np | C 1/IOD(0.2%/2%) | 39.6 | 0.71 |
| BBS/IOD(2%/3%) | np | np | Ir369(1%) | 34.5 | 0.39 |
| OB 7/IOD(1%/3%) | 39.0 | 0.59 | Ir819(1%) | 38.0 | 0.82 |
| CBUS 450/IOD(2%/3%) | 19.2 | 0.05 |
| PIS | FC(%) | PR | PIS | FC(%) | PR |
|---|---|---|---|---|---|
| OBs alone | np* | np | CBS X/IOD(0.2%/1%) | 50.7 | 0.71 |
| IOD alone | np | np | C 1/IOD(0.2%/2%) | 39.6 | 0.71 |
| BBS/IOD(2%/3%) | np | np | Ir369(1%) | 34.5 | 0.39 |
| OB 7/IOD(1%/3%) | 39.0 | 0.59 | Ir819(1%) | 38.0 | 0.82 |
| CBUS 450/IOD(2%/3%) | 19.2 | 0.05 |
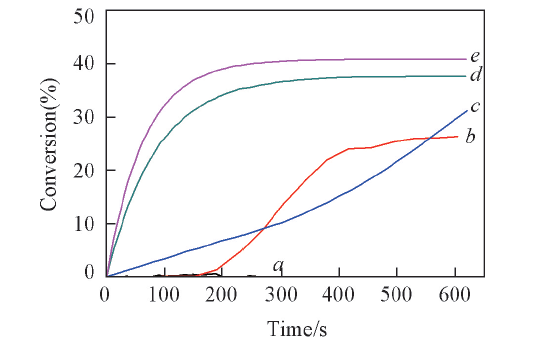
Fig.7 Photopolymerization profiles(acrylate function conversion vs. time) of Ebecryl 605/TMPTA(mass ratio, 70/30) blends under air and exposure to LED@420 nm in the presence of BBS/IOD/NVK(a), BBS/IOD/EDB(b), OB 7/IOD(c), OB 7/IOD/NVK(d), and OB 7/IOD/EDB(e)
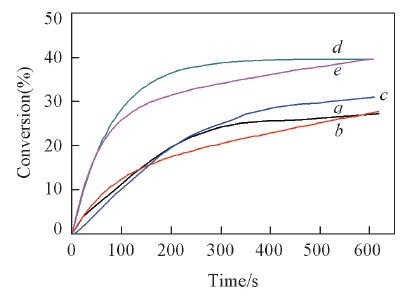
Fig.8 Photopolymerization profiles(acrylate function conversion vs. time) of Ebecryl 605/TMPTA(mass ratio, 70/30) blends under air and exposure to LED@420 nm in the presence of CBUS 450/IOD/NVK(a), CBUS 450/IOD/EDB(b), CBS X/IOD(c), CBS X/IOD/NVK(d) and CBS X/IOD/EDB(e)
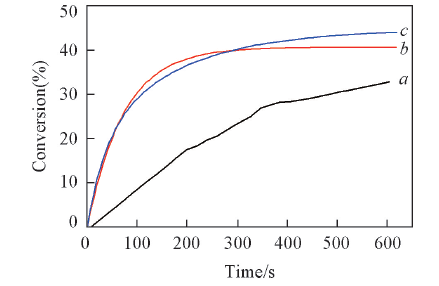
Fig.9 Photopolymerization profiles(acrylate function conversion vs. time) of Ebecryl 605/TMPTA(mass ratio, 70/30) blends under air and exposure to LED@420 nm in the presence of C 1/IOD(a), C 1/IOD/NVK(b) and C 1/IOD/EDB(c)
| PISs | None | 2%NVK | 2%EDB | |||
|---|---|---|---|---|---|---|
| FC | RP | FC | RP | FC | RP | |
| OB alone | | | np* | np | np | np |
| BBS/IOD(2%/3%) | np | np | np | np | 26.3 | 0.11 |
| OB 7/IOD(1%/3%) | 31.2 | 0.08 | 37.7 | 0.43 | 40.9 | 0.61 |
| CBUS 450/IOD(2%/3%) | 19.2 | 0.05 | 27.3 | 0.18 | 27.7 | 0.20 |
| CBS X/IOD(0.2%/1%) | 31.6 | 0.11 | 39.6 | 0.48 | 39.7 | 0.53 |
| C 1/IOD(0.2%/2%) | 32.7 | 0.09 | 40.6 | 0.54 | 44.0 | 0.63 |
| PISs | None | 2%NVK | 2%EDB | |||
|---|---|---|---|---|---|---|
| FC | RP | FC | RP | FC | RP | |
| OB alone | | | np* | np | np | np |
| BBS/IOD(2%/3%) | np | np | np | np | 26.3 | 0.11 |
| OB 7/IOD(1%/3%) | 31.2 | 0.08 | 37.7 | 0.43 | 40.9 | 0.61 |
| CBUS 450/IOD(2%/3%) | 19.2 | 0.05 | 27.3 | 0.18 | 27.7 | 0.20 |
| CBS X/IOD(0.2%/1%) | 31.6 | 0.11 | 39.6 | 0.48 | 39.7 | 0.53 |
| C 1/IOD(0.2%/2%) | 32.7 | 0.09 | 40.6 | 0.54 | 44.0 | 0.63 |
| Monomer | BBS/IOD (2%/3%) | OB 7/IOD (1%/3%) | CBUS 450/ IOD(2%/3%) | CBS X/ IOD(0.2%/1%) | C 1/IOD (0.2%/2%) |
|---|---|---|---|---|---|
| EPOX | np* | 29.7 | 25.7 | 36.4 | 29.5 |
| Ebecryl 605 | np | 54.6 | 51.6 | 68.0 | 63.7 |
| Monomer | BBS/IOD (2%/3%) | OB 7/IOD (1%/3%) | CBUS 450/ IOD(2%/3%) | CBS X/ IOD(0.2%/1%) | C 1/IOD (0.2%/2%) |
|---|---|---|---|---|---|
| EPOX | np* | 29.7 | 25.7 | 36.4 | 29.5 |
| Ebecryl 605 | np | 54.6 | 51.6 | 68.0 | 63.7 |
| [1] | Crivello J V., . Dietliker K., Photoinitiators for Free Radical, In Cationic and Anionic Photopolymerization, Wiley, Chichester, 1998, 122— 140 |
| [2] | Liang B., Kuang S. J., Huang J. J., Man L. L., Yang Z. H., Yuan T., Prog. Org.Coat., 2019, 129, 116— 124 |
| [3] | Fouassier J P., .Lalevée J., Photoinitiators for Polymer Synthesis, Scope, Reactivity and Efficiency, Wiley-VCH., Weinheim, Berlin, 2012, 560— 589 |
| [4] | Dietlin C., Schweizer S., Xiao P., Zhang J., Morlet-Savary F., Graff B., Fouassier J. P., Lalevée J., Polym. Chem., 2015, 6( 21), 3895— 3912 |
| [5] | Lalevée J., Blanchard N., Tehfe M. A., Morlet-Savary F., Fouassier J. P ., Macromolecules, 2010, 43( 24), 10191— 10195 |
| [6] | Shao J. H., Huang Y., Fan Q. G., Polym.Chem, 2014, 5( 14), 4195— 4210 |
| [7] | Tehfe M. A., Lalevée J., Morlet-Savary F., Graff B., Blanchard N., Fouassier J. P ., Macromolecules, 2012, 45( 4), 1746— 1752 |
| [8] | Ligon S. C., Husár B., Wutzel H., Holman R., Liska R., Chem. Rev., 2014, 114( 1), 557— 589 |
| [9] | Xiao P., Dumur F., Graff B., Gigmes D., Fouassier J. P ., Macromolecules, 2013, 46( 19), 7661— 7667 |
| [10] | Allen N. S. J., Photochem. Photobiol. A., 1996, 100( 1—3), 101— 107 |
| [11] | Bi Y., Neckers D. C ., Macromolecules, 1994, 27( 14), 3683— 3693 |
| [12] | Xiao P., Zhang J., Dumur F., Tehfe M. A., Morlet-Savary F., Graffa B., Gigmes D., Fouassier J. P., Lalevée J., Prog. Polym. Sci., 2015, 41, 32— 66 |
| [13] | Ji S., Wu W., Song P., Han K., Wang Z., Liu S., Guo H., Zhao J., J. Mater. Chem., 2010, 20( 10), 1953— 1963 |
| [14] | Lalevée J., Dumur F., Mayer C. R., Gigmes D., Nasr G., Tehfe M. A., Telitel S., Morlet-Savary F., Graff B., Fouassier J. P ., Macromolecules, 2012, 45( 10), 4134— 4141 |
| [15] | Chen R. Y., Qu J. G., Zhao Q. Q., He J. X., Fiber Polym., 2014, 15( 9), 1915— 1920 |
| [16] | Zhao Q. Q., Sun J., Liu B. J., He J. X ., Cellulose, 2014, 21( 4), 2937— 2950 |
| [17] | Zuo X. L., Morlet-Savary F., Graff B., Blanchard N., Goddard J. P., Lalevée J., Macromol. Rapid Comm., 2016, 37( 10), 840— 844 |
| [18] | Lalevée J., Tehfe M. A., Zein-Fakih A., Ball B., Telitel S., Morlet-Savary F., Graff B., Fouassier J. P., ACS Macro Lett., 2012, 1( 7), 802— 806 |
| [19] | Xiao P., Dumuí F., Graff B., Morlet-Savary F., Gigmes D., Fouassier J. P., Lalevée J ., Macromolecules, 2014, 47( 3), 973— 978 |
| [20] | Telitel S., Schweizer S., Morlet-Savary F., Graff B., Tschamber T., Blanchard N., Morlet-Savary F., Lelli M., Lacôte E., Lalevée J ., Macromolecules, 2013, 46( 1), 43— 48 |
| [21] | Tehfe M. A., Lalevée J., Telitel S., Sun J., Zhao Z., Graff B., Morlet-Savary F., Fouassier J. P ., Polymer, 2012, 53( 14), 2803— 2808 |
| [22] | Fourati M. A., Maris T., Skene W. G., Bazuin C. G.Prud’homme R. E., J. Phys. Chem. B, 2011, 115( 43), 12362— 12369 |
| [23] | Jones II G., Jackson W. R., Choi C., Bergmark W. R., J. Phys. Chem., 1985, 89( 2), 294— 300 |
| [24] | Zuo X. L., Morlet-Savary F., Schmitt M., Le Nouën D., Blanchard N., Goddard J. P., Lalevée J., Polym. Chem., 2018, 9( 28), 3952— 3958 |
| [25] | Gao F., Chin. J. Polym.Sci., 1999, 6, 589— 594 |
| ( 高放 . 高分子科学, 1999 6, 589— 594) | |
| [26] | Chen R. Y., Qu J. G., Yang D., He J. X., Text. Res.J., 2014, 84( 7), 772— 782 |
| [27] | Grabchev I., Philipova T., Dyes Pigments , 2000, 44( 3), 175— 180 |
| [1] | LI Xingjian, WU Ruiqing, LAI Jingjuan, PAN Yi, ZHENG Zhaohui, DING Xiaobin. Shape-memory Properties and Molecular Mechanism of Poly(methyl methacrylate)/Star-shaped Poly(ethylene glycol) Semi-interpenetrating Polymer Network† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1932. |
| [2] | LIANG Shuang, YANG Jian-Hai, LIU Gui-Pei, LIU Wen-Guang*. Corneal Substitutes of Protein-loaded Collagen/Polyzwitterion IPN [J]. Chem. J. Chinese Universities, 2010, 31(4): 821. |
| [3] | WU Wen, WANG Dong-Sheng, WANG Li-Qun*. Synthesis and Characterization of Fast pH-Responsive Silk Sericin/Poly(methacrylic acid) Interpenetrating Polymer Network Hydrogel [J]. Chem. J. Chinese Universities, 2009, 30(4): 830. |
| [4] | XIE Hong-Quan, HUANG Xu-Dong, GUO Jun-Shi . Complexes of Interpenetrating Polymer Networks Exhibiting Synergistic Effect of Ionic and Electronic Conductivities [J]. Chem. J. Chinese Universities, 2004, 25(5): 957. |
| [5] | DONG Han-Peng, LI Xiao-Yu . Studies on Acrylate Thermoplastic Elastomer with Latex Interpenetrating Polymer Network Structure [J]. Chem. J. Chinese Universities, 2004, 25(4): 774. |
| [6] | XIAO Shan-Qiang, CHEN Qi-Dao, CHEN Ming, HONG Xiao-Yin. Studies on Epoxy-acrylate Hybrid UV-cure System [J]. Chem. J. Chinese Universities, 2002, 23(9): 1797. |
| [7] | LI Xiao-Yu, SHI Shu-Xian, KOU Yi, DONG Han-Peng, XIA Yu-Zheng . Preparation of Mosaic Latex Particles [J]. Chem. J. Chinese Universities, 2001, 22(1): 146. |
| [8] | HUA Feng-Jun, LI Jun, HU Chun-Pu, LIU Hong-Lai, HU Ying . Diffusion Behavior of Ethanol in Epoxy/Urethane Acrylate Resin Interpenetrating Polymer Network (Ⅱ)——Heterogeneous Transport Model and Application [J]. Chem. J. Chinese Universities, 1998, 19(12): 1987. |
| [9] | HUA Feng-Jun, LI Jun, HU Chun-Pu, LIU Hong-Lai, HU Ying . Diffusion Behavior of Ethanol in Epoxy/Urethane Acrylate Resin Interpenetrating Polymer Networks(Ⅰ)——Homogeneous Transport Model and Correlation with Experimental Sorption Curves [J]. Chem. J. Chinese Universities, 1998, 19(11): 1807. |
| [10] | HAN Qing-Guo, WANG Jing-Yuan, LIU Rui-Ying, LI Yu-Wei, TANG Xin-Yi . The Effect of Crosslinking Density on Dynamic Mechanical Properties of Polyurethane/Polystyrene Interpenetrating Polymer Networks [J]. Chem. J. Chinese Universities, 1995, 16(4): 653. |
| [11] | CHEN Bao-Quan, HAN Xiao-Zu, GUO Feng-Chun . Studies on Polyepichlorohydrin-Based Polyurethane/ Poly(MMA-co-St)IPNs [J]. Chem. J. Chinese Universities, 1995, 16(10): 1637. |
| [12] | WANGYong-Jian, LIN Xue, YANG Yi-Zhong, HE Bing-Lin. Investigation of Interpenetrating Polymer Network Chelate Resin(Ⅰ)─Syntheses of Crosslinked Polystyrene-Polyacrylonitrile Interpenetrating Polymer Network [J]. Chem. J. Chinese Universities, 1994, 15(9): 1408. |
| [13] | LUO Xiao-Lie, LIU Jin, FAN Zhong-Dong, MA De-Zhu, ZNAO Chun-Tian, HOU Jian-An, CUI Di, XU Mao. Synthesis and Characterization of a Novel Interpenetrating Polymer Network PP/PnBA [J]. Chem. J. Chinese Universities, 1994, 15(10): 1553. |
| [14] | XIAO Hong, JIANG Ming, YU Tong-Yin . Interpenetrating Polymer Networks with Introduced Hydrogen Bonding ( Ⅴ ) ——The Effecct of Composition on Phase Structure and the Properties [J]. Chem. J. Chinese Universities, 1993, 14(8): 1167. |
| [15] | LI YAO-Xian, LIU Fu-An, WANG Jing-Yuan, LI Yu-Wei, TANG Xin-Yi . The Synthesis and Characterization of Chlorosulfonated Polyethyl ene/Pol ybutyl Methacrylate/Epoxy Resins Three-Component IPNs [J]. Chem. J. Chinese Universities, 1992, 13(8): 1141. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||