

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (10): 1778.doi: 10.7503/cjcu20170094
• Organic Chemistry • Previous Articles Next Articles
ZHANG Lingzhi1, JIANG Minrui2, WEI Ping3, ZHU Qihua1,*( ), GONG Guoqing2, BIAN Xueguo3, XU Yungen1,*(
), GONG Guoqing2, BIAN Xueguo3, XU Yungen1,*( )
)
Received:2017-02-18
Online:2017-10-10
Published:2017-09-22
Contact:
ZHU Qihua,XU Yungen
E-mail:zhuqihua@vip.126.com;xyg@cpu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Lingzhi, JIANG Minrui, WEI Ping, ZHU Qihua, GONG Guoqing, BIAN Xueguo, XU Yungen. Synthesis, Metabolic Stability and Biological Activity in vivo of Lorcaserin Derivatives†[J]. Chem. J. Chinese Universities, 2017, 38(10): 1778.
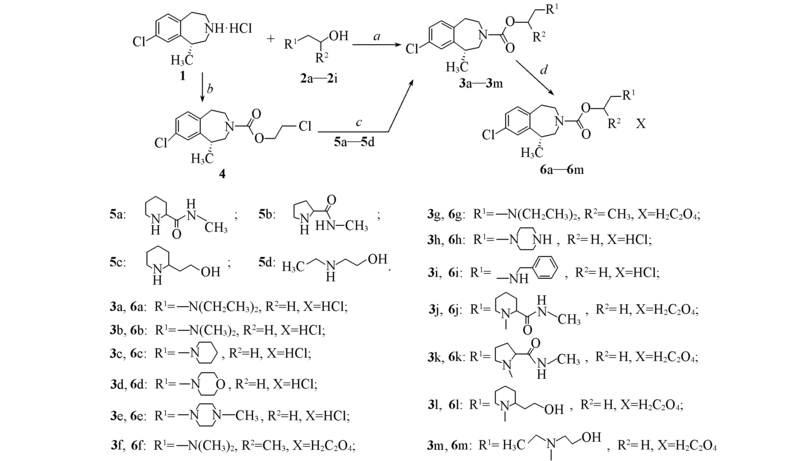
Scheme 1 Synthetic routes of compounds 6a—6mRegents and conditions: a. (i) CDI, dry CH2Cl2, N2, 0—20 ℃, 5 h, (ii) lorcaserin hydrochloride, triethylamine, r. t., CH2Cl2, overnight, (iii) CF3COOH, r. t., 30 min(3h and 3i); b. 2-chloroethyl chloroformate, triethylamine, dry CH2Cl2, 0 ℃, 4 h; c. K2CO3, NaI, 5a—5d; d. HCl, dry CH2Cl2 or H2C2O4, dry acetone.
| Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) |
|---|---|---|---|---|---|---|---|---|
| 3a | Yellow oil | 80.4 | 3f | Yellow oil | 81.7 | 3j | Yellow oil | 72.5 |
| 3b | Yellow oil | 38.6 | 3g | Yellow oil | 85.8 | 3k | Yellow oil | 87.1 |
| 3c | Yellow oil | 42.3 | 3h | Yellow oil | 37.5 | 3l | Yellow oil | 78.1 |
| 3d | Yellow oil | 42.0 | 3i | Yellow oil | 81.5 | 3m | Yellow oil | 45.5 |
| 3e | Yellow oil | 43.8 |
Table 1 Appearances and yields of compounds 3a—3m*
| Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) |
|---|---|---|---|---|---|---|---|---|
| 3a | Yellow oil | 80.4 | 3f | Yellow oil | 81.7 | 3j | Yellow oil | 72.5 |
| 3b | Yellow oil | 38.6 | 3g | Yellow oil | 85.8 | 3k | Yellow oil | 87.1 |
| 3c | Yellow oil | 42.3 | 3h | Yellow oil | 37.5 | 3l | Yellow oil | 78.1 |
| 3d | Yellow oil | 42.0 | 3i | Yellow oil | 81.5 | 3m | Yellow oil | 45.5 |
| 3e | Yellow oil | 43.8 |
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
|---|---|
| 3a | 7.13—7.11(m, 1H), 7.08(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—6.99(m, 1H), 4.16(t, J=6.2 Hz, 2H), 3.84—3.33(m, 4H), 3.13—3.96(m, 2H), 2.81(dd, J=15.1, 6.2 Hz, 1H), 2.70(t, J=6.3 Hz, 2H), 2.58(q, J=7.1 Hz, 4H), 1.30—1.24(m, 3H), 1.03(t, J=7.1 Hz, 6H) |
| 3b | 7.13—7.10(m, 1H), 7.09(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—7.01(m, 1H), 4.20(t, J=5.9 Hz, 2H), 3.77—3.35(m, 4H), 3.03—3.00(m, 2H), 2.84—2.80(m, 1H), 2.56(t, J=5.9 Hz, 2H), 2.28(s, 6H), 1.30—1.24(m, 3H) |
| 3c | 7.14—7.07(m, 2H), 7.03—7.00(m, 1H), 4.23(t, J=6.0 Hz, 2H), 3.76—3.42(m, 4H), 3.08—3.00(m, 2H), 2.82(dd, J1=15.1 Hz, J2= 6.5 Hz, 1H), 2.61(t, J=5.9 Hz, 2H), 2.46—2.44(m, 4H), 1.60—1.55(m, 4H), 1.46—1.43(m, 2H), 1.30—1.28(m, 3H) |
| 3d | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 4.23(t, J=5.8 Hz, 2H), 3.76—3.72(m, 1H), 3.70(t, J=4.5 Hz, 4H), 3.64—3.36(m, 3H), 3.08—3.00(m, 2H), 2.83(dd, J1=14.3 Hz, J2=4.7 Hz, 1H), 2.63(t, J=5.8 Hz, 2H), 2.52(t, J=4.6 Hz, 4H), 1.29(t, J=7.0 Hz, 3H) |
| 3e | 7.13—7.07(m, 2H), 7.03—7.01(m, 1H), 4.22(t, J=5.9 Hz, 2H), 3.74—3.40(m, 4H), 3.07—2.96(m, 2H), 2.82(dd, J1=15.2 Hz, J2=4.0 Hz, 1H), 2.64(t, J=5.9 Hz, 2H), 2.55—2.44(m, 8H), 2.28(s, 3H), 1.29—1.27(m, 3H) |
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
| 3f | 7.12—7.07(m, 2H), 7.01—6.98(m, 1H), 5.00—4.97(m, 1H), 3.84—3.34(m, 4H), 3.06—3.02(m, 2H), 2.84(dd, J1=14.0 Hz, J2=5.5 Hz, 1H), 2.56—2.41(m, 1H), 2.33—2.29(m, 1H), 2.27(s, 3H), 2.25(s, 3H), 1.30(d, J=6.9 Hz, 3H), 1.22(d, J=6.2 Hz, 3H) |
| 3g | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 5.00—4.91(m, 1H), 3.65—3.43(m, 4H), 3.09—3.01(m, 2H), 2.82(dd, J1=15.0 Hz, J2=6.5 Hz, 1H), 2.64—2.50(m, 5H), 2.45—2.40(m, 1H), 1.30(d, J=7.1 Hz, 3H), 1.22(d, J=6.3 Hz, 3H), 1.01(td, J1=7.1 Hz, J2=2.8 Hz, 6H) |
| 3h | 7.17—7.14(m, 1H), 7.11(dd, J1=8.1 Hz, J2=2.0 Hz, 1H), 7.04—7.03(m, 1H), 4.23(t, J=5.9 Hz, 2H), 3.78—3.41(m, 4H), 3.08—3.00(m, 2H), 2.96—2.91(m, 2H), 2.84(dd, J1=14.5 Hz, J2=5.2 Hz, 1H), 2.65—2.61(m, 4H), 2.56—2.46(m, 4H), 1.31—1.28(m, 3H), 1.25(s, 1H) |
| 3i | 7.35—7.27(m, 5H), 7.15—7.08(m, 2H), 7.04—6.99(m, 1H), 4.27—4.23(t, J=5.4 Hz, 2H), 3.85(s, 2H), 3.65—3.39(m, 4H), 3.09—3.01(m, 2H), 2.90(t, J=5.3 Hz, 2H), 2.82(dd, J1=15.4 Hz, J2=6.5 Hz, 1H), 1.88(s, 1H), 1.31—1.2(m, 3H) |
| 3j | 7.15—7.10(m, 2H), 7.05—7.02(m, 1H), 6.79(s, 1H), 4.28—4.17(m, 2H), 3.81—3.40(m, 4H), 3.11—2.96(m, 3H), 2.87—2.82(m, 6H), 2.45—2.40(m, 1H), 2.15—2.12(m, 1H), 1.98—1.93(m, 2H), 1.72—1.63(m, 2H), 1.51—1.24(m, 2H), 1.33—1.28(m, 3H) |
| 3k | 7.45(s, 1H), 7.16—7.11(m, 2H), 7.05—7.00(m, 1H), 4.20—4.12(m, 2H), 3.74—3.30(m, 4H), 3.28—3.02(m, 3H), 2.99—2.70(m, 6H), 2.60—2.36(m, 1H), 2.26—2.08(m, 1H), 1.92—1.78(m, 4H), 1.34—1.28(m, 3H) |
| 3l | 7.14—7.09(m, 2H), 7.04—7.01(m, 1H), 4.21(t, J=5.8 Hz, 2H), 3.91—3.88(m, 4H), 3.22—2.94(m, 4H), 2.87—2.77(m, 4H), 2.44—2.40(m, 1H), 2.00—1.98(m, 1H), 1.73—1.53(m, 3H), 1.51—1.43(m, 3H), 1.32—1.26(m, 3H) |
| 3m | 7.18—7.08(m, 2H), 7.07—7.00(m, 1H), 4.21—4.18(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.84(dd, J1=15.1 Hz, J2=5.9 Hz, 1H), 1.30—1.29(m, 3H), 1.29(t, J=6.8 Hz, 3H) |
Table 2 1H NMR data of compounds 3a—3m
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
|---|---|
| 3a | 7.13—7.11(m, 1H), 7.08(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—6.99(m, 1H), 4.16(t, J=6.2 Hz, 2H), 3.84—3.33(m, 4H), 3.13—3.96(m, 2H), 2.81(dd, J=15.1, 6.2 Hz, 1H), 2.70(t, J=6.3 Hz, 2H), 2.58(q, J=7.1 Hz, 4H), 1.30—1.24(m, 3H), 1.03(t, J=7.1 Hz, 6H) |
| 3b | 7.13—7.10(m, 1H), 7.09(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—7.01(m, 1H), 4.20(t, J=5.9 Hz, 2H), 3.77—3.35(m, 4H), 3.03—3.00(m, 2H), 2.84—2.80(m, 1H), 2.56(t, J=5.9 Hz, 2H), 2.28(s, 6H), 1.30—1.24(m, 3H) |
| 3c | 7.14—7.07(m, 2H), 7.03—7.00(m, 1H), 4.23(t, J=6.0 Hz, 2H), 3.76—3.42(m, 4H), 3.08—3.00(m, 2H), 2.82(dd, J1=15.1 Hz, J2= 6.5 Hz, 1H), 2.61(t, J=5.9 Hz, 2H), 2.46—2.44(m, 4H), 1.60—1.55(m, 4H), 1.46—1.43(m, 2H), 1.30—1.28(m, 3H) |
| 3d | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 4.23(t, J=5.8 Hz, 2H), 3.76—3.72(m, 1H), 3.70(t, J=4.5 Hz, 4H), 3.64—3.36(m, 3H), 3.08—3.00(m, 2H), 2.83(dd, J1=14.3 Hz, J2=4.7 Hz, 1H), 2.63(t, J=5.8 Hz, 2H), 2.52(t, J=4.6 Hz, 4H), 1.29(t, J=7.0 Hz, 3H) |
| 3e | 7.13—7.07(m, 2H), 7.03—7.01(m, 1H), 4.22(t, J=5.9 Hz, 2H), 3.74—3.40(m, 4H), 3.07—2.96(m, 2H), 2.82(dd, J1=15.2 Hz, J2=4.0 Hz, 1H), 2.64(t, J=5.9 Hz, 2H), 2.55—2.44(m, 8H), 2.28(s, 3H), 1.29—1.27(m, 3H) |
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
| 3f | 7.12—7.07(m, 2H), 7.01—6.98(m, 1H), 5.00—4.97(m, 1H), 3.84—3.34(m, 4H), 3.06—3.02(m, 2H), 2.84(dd, J1=14.0 Hz, J2=5.5 Hz, 1H), 2.56—2.41(m, 1H), 2.33—2.29(m, 1H), 2.27(s, 3H), 2.25(s, 3H), 1.30(d, J=6.9 Hz, 3H), 1.22(d, J=6.2 Hz, 3H) |
| 3g | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 5.00—4.91(m, 1H), 3.65—3.43(m, 4H), 3.09—3.01(m, 2H), 2.82(dd, J1=15.0 Hz, J2=6.5 Hz, 1H), 2.64—2.50(m, 5H), 2.45—2.40(m, 1H), 1.30(d, J=7.1 Hz, 3H), 1.22(d, J=6.3 Hz, 3H), 1.01(td, J1=7.1 Hz, J2=2.8 Hz, 6H) |
| 3h | 7.17—7.14(m, 1H), 7.11(dd, J1=8.1 Hz, J2=2.0 Hz, 1H), 7.04—7.03(m, 1H), 4.23(t, J=5.9 Hz, 2H), 3.78—3.41(m, 4H), 3.08—3.00(m, 2H), 2.96—2.91(m, 2H), 2.84(dd, J1=14.5 Hz, J2=5.2 Hz, 1H), 2.65—2.61(m, 4H), 2.56—2.46(m, 4H), 1.31—1.28(m, 3H), 1.25(s, 1H) |
| 3i | 7.35—7.27(m, 5H), 7.15—7.08(m, 2H), 7.04—6.99(m, 1H), 4.27—4.23(t, J=5.4 Hz, 2H), 3.85(s, 2H), 3.65—3.39(m, 4H), 3.09—3.01(m, 2H), 2.90(t, J=5.3 Hz, 2H), 2.82(dd, J1=15.4 Hz, J2=6.5 Hz, 1H), 1.88(s, 1H), 1.31—1.2(m, 3H) |
| 3j | 7.15—7.10(m, 2H), 7.05—7.02(m, 1H), 6.79(s, 1H), 4.28—4.17(m, 2H), 3.81—3.40(m, 4H), 3.11—2.96(m, 3H), 2.87—2.82(m, 6H), 2.45—2.40(m, 1H), 2.15—2.12(m, 1H), 1.98—1.93(m, 2H), 1.72—1.63(m, 2H), 1.51—1.24(m, 2H), 1.33—1.28(m, 3H) |
| 3k | 7.45(s, 1H), 7.16—7.11(m, 2H), 7.05—7.00(m, 1H), 4.20—4.12(m, 2H), 3.74—3.30(m, 4H), 3.28—3.02(m, 3H), 2.99—2.70(m, 6H), 2.60—2.36(m, 1H), 2.26—2.08(m, 1H), 1.92—1.78(m, 4H), 1.34—1.28(m, 3H) |
| 3l | 7.14—7.09(m, 2H), 7.04—7.01(m, 1H), 4.21(t, J=5.8 Hz, 2H), 3.91—3.88(m, 4H), 3.22—2.94(m, 4H), 2.87—2.77(m, 4H), 2.44—2.40(m, 1H), 2.00—1.98(m, 1H), 1.73—1.53(m, 3H), 1.51—1.43(m, 3H), 1.32—1.26(m, 3H) |
| 3m | 7.18—7.08(m, 2H), 7.07—7.00(m, 1H), 4.21—4.18(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.84(dd, J1=15.1 Hz, J2=5.9 Hz, 1H), 1.30—1.29(m, 3H), 1.29(t, J=6.8 Hz, 3H) |
| Compd. | Appearance | m. p. /℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 6a | White solid | 156—158 | 78.2 | 339.1834(339.1840) |
| 6b | White solid | 150—152 | 37.5 | 311.1521(311.1529) |
| 6c | White solid | 152—154 | 41.2 | 351.1834(351.1841) |
| 6d | White solid | 157—158 | 41.0 | 353.1626(353.1625) |
| 6e | White solid | 222—224 | 42.4 | 366.1943(366.1949) |
| 6f | White solid | 103—105 | 78.7 | 325.1677(325.1674) |
| 6g | White solid | 98—100 | 82.4 | 353.1990(353.1985) |
| 6h | White solid | 178—180 | 36.4 | 352.1786(352.1795) |
| 6i | White solid | 178—180 | 79.0 | 373.1677(373.1674) |
| 6j | White solid | 85—86 | 69.9 | 408.2048(408.2045) |
| 6k | White solid | 127—129 | 84.2 | 394.1892(394.1892) |
| 6l | White solid | 75.1 | 395.2096(395.2091) | |
| 6m | White solid | 43.7 | 355.1783(355.1782) |
Table 3 Appearances, melting points, yield and HRMS data of compounds 6a—6m*
| Compd. | Appearance | m. p. /℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 6a | White solid | 156—158 | 78.2 | 339.1834(339.1840) |
| 6b | White solid | 150—152 | 37.5 | 311.1521(311.1529) |
| 6c | White solid | 152—154 | 41.2 | 351.1834(351.1841) |
| 6d | White solid | 157—158 | 41.0 | 353.1626(353.1625) |
| 6e | White solid | 222—224 | 42.4 | 366.1943(366.1949) |
| 6f | White solid | 103—105 | 78.7 | 325.1677(325.1674) |
| 6g | White solid | 98—100 | 82.4 | 353.1990(353.1985) |
| 6h | White solid | 178—180 | 36.4 | 352.1786(352.1795) |
| 6i | White solid | 178—180 | 79.0 | 373.1677(373.1674) |
| 6j | White solid | 85—86 | 69.9 | 408.2048(408.2045) |
| 6k | White solid | 127—129 | 84.2 | 394.1892(394.1892) |
| 6l | White solid | 75.1 | 395.2096(395.2091) | |
| 6m | White solid | 43.7 | 355.1783(355.1782) |
| Compd. | 1H NMR(300 MHz), δ* | 13C NMR(75 MHz), δ* |
|---|---|---|
| 6a | 12.55(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.04(m, 1H), 4.62(t, J=5.0 Hz, 2H), 3.86—3.47(m, 4H), 3.28—3.02(m, 8H), 2.89—2.86(dd, J1=15.7Hz, J2=4.6 Hz, 1H), 1.47—1.37(m, 6H), 1.32—1.29(m, 3H) | 154.91, 145.40, 136.95, 131.18, 127.77, 126.38, 115.18, 59.16, 51.72, 49.69, 47.32, 46.34, 40.57, 35.11, 17.09, 8.32 |
| 6b | 12.66(bs, 1H), 7.13—7.11(m, 2H), 7.04—7.01(m, 1H), 4.62—4.55(m, 2H), 3.82—3.45(m, 4H), 3.33—3.19(m, 2H), 3.14—3.01(m, 2H), 2.96—2.75(m, 7H), 1.30(d, J=7.0 Hz, 3H) | 154.37, 145.07, 136.17, 132.42, 130.70, 128.18, 125.23, 58.94, 55.51, 49.64, 45.39, 42.86, 39.52, 34.88, 17.59 |
| 6c | 12.45(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.03(m, 1H), 4.65—4.64(m, 2H), 3.82—3.45(m, 6H), 3.24—3.22(m, 2H), 3.18—3.00(m, 2H), 2.88—2.83(m, 1H), 2.70—2.63(m, 2H), 2.33—2.30(m, 2H), 1.95—1.85(m, 3H), 1.50—1.37(m, 1H), 1.33—1.29(m, 3H) | 155.25, 145.55, 136.66, 132.35, 131.76, 127.85, 125.73, 59.43, 56.01, 53.48, 51.44, 46.30, 40.03, 34.97, 22.25, 21.44, 17.20 |
| 6d | 13.39(s, 1H), 7.15—7.11(m, 2H), 7.06—7.02(m, 1H), 4.64—4.40(m, 2H), 4.36—4.28(m, 2H), 4.01—3.98(m, 2H ), 3.81—3.50(m, 4H), 3.48—3.39(m, 2H), 3.30—3.22(m, 2H), 3.17—3.03(m, 2H), 2.99—2.89(m, 3H), 1.31—1.28(m, 3H) | 154.87, 145.75, 137.16, 132.16, 131.47, 128.06, 126.38, 63.92, 58.78, 56.01, 51.69, 50.95, 45.81, 39.95, 34.80, 17.11 |
| 6e | 7.15(d,J=1.9 Hz, 1H), 7.07(dd, J1=8.1 Hz, J2=1.9 Hz, 1H), 7.02(d, J=8.1 Hz, 1H), 4.19—4.17(m, 2H), 3.60—3.55(m, 4H), 3.44—3.24(m, 10H ), 3.12—3.06(m, 2H), 2.92(s, 3H), 2.79—2.74(m, 1H), 1.12(d, J=7.1 Hz, 3H) | 156.37, 145.13, 137.06, 131.19, 127.85, 126.14, 114.72, 59.43, 56.01, 50.96, 49.33, 48.83, 45.48, 42.55, 39.12, 33.26, 16.38 |
| 6f | 9.01(bs, 2H), 7.14—7.11(m, 2H), 7.07—7.03(m, 1H), 5.26—5.20(m, 1H), 3.84—3.48(m, 4H), 3.44—3.30(m, 2H), 3.12—3.00(m, 3H), 2.89(d, J=6.6 Hz, 3H),2.84(d, J=4.0 Hz, 3H), 1.31—1.29(m, 3H), 1.16(d, J=6.1 Hz, 3H) | 162.83, 154.43, 145.13, 136.66, 132.41, 127.84, 126.15, 68.73, 65.31, 60.25, 50.95, 46.29, 45.48, 42.55, 35.78, 22.25, 16.78 |
| 6g | 12.18(bs, 2H),7.16—7.12(m, 2H), 7.08—7.03(m, 1H),5.27—5.21(m, 1H), 3.78—3.44(m, 4H), 3.41—3.23(m, 6H), 3.07—2.93(m, 3H), 1.36—1.27(m, 12H) | 155.65, 145.96, 137.96, 132.01, 127.86, 126.14, 115.13, 72.97, 70.37, 58.12, 50.95, 47.60, 45.49, 40.44, 36.19, 18.91,17.35 11.73 |
| 6h | 7.11(m, 1H),7.05—6.98(m, 2H), 4.19—4.17(m, 2H), 3.60—3.57(m, 1H), 3.41—3.38(m, 6H), 3.25—3.15(m, 7H), 3.08—3.02(m, 1H), 2.96—2.88(m, 1H), 2.77—2.69(m, 1H), 1.10(d, J=7.1 Hz, 3H) | 145.95, 137.06, 132.71, 131.99, 127.84, 126.13, 114.72, 59.84, 56.00, 50.54, 48.43, 45.08, 41.66, 38.31, 33.25, 15.97. |
| 6i | 10.25(bs, 2H), 7.64—7.62(m, 2H), 7.43—7.41(m, 3H), 7.15—7.09(m, 2H), 7.04—7.01(m, 1H), 4.50—4.69(m, 2H), 4.19(s, 2H), 3.83—3.80(m, 1H), 3.60—3.55(m, 3H), 3.11—3.02(m, 4H), 2.91—2.79(m, 1H), 1.30—1.25(m, 3H) | 155.51, 145.80, 136.60, 131.30, 129.78, 129.41, 129.15, 128.79, 127.50, 125.82, 125.73, 59.90, 51.05, 49.99, 46.18, 44.92, 40.39, 35.47,17.17 |
| 6j | 11.49(bs, 0.5H), 10.83(bs, 0.5H), 9.11(bs, 0.5H), 8.79(bs, 0.5H), 7.15—7.11(m, 2H), 7.07—7.03(m, 1H), 4.60—4.37(m, 3H), 3.76—3.34(m, 7H), 3.21—3.01(m, 3H), 2.83(s, 3H), 2.72—2.61(m, 1H), 2.40—2.09(m, 3H), 1.99—1.81(m, 2H), 1.75—1.59(m, 1H), 1.36—1.30(m, 3H) | 175.06, 155.65, 145.13, 137.96, 132.00, 128.66, 126.13, 114.72, 67.84, 62.77, 55.20, 51.85, 51.30, 46.79, 41.38, 40.84, 36.60, 29.02, 25.68, 24.78, 23.15, 17.61 |
| 6k | 7.15—7.12(m, 1H), 7.09—7.01(m, 2H), 4.19—4.14(m, 2H), 3.69—3.63(m, 2H ), 3.53—3.36(m, 5H), 3.23—3.02(m, 3H), 3.02—2.88(m, 1H), 2.85—2.71(m, 1H), 2.63(d, J =5.6 Hz, 3H), 2.51—2.32(m, 1H), 2.09—1.93(m, 3H), 1.12(d, J=6.5 Hz, 3H) | 167.89, 156.56, 146.36, 137.06, 132.00, 127.85, 126.55, 115.13, 67.42, 64.49, 60.25, 56.50, 50.13, 46.31, 39.14, 32.85, 30.15, 29.42, 26.49, 22.25, 16.38 |
| 6l | 7.13—7.09(m, 2H), 7.05—7.02(m, 1H), 4.50—4.18(m, 2H), 3.91—3.30(m, 10H), 3.23—3.01(m, 3H), 2.86—2.81(m, 1H), 2.09—1.82(m, 8H), 1.33—1.25(m, 3H) | 156.06, 146.36, 137.97, 132.41, 128.25, 126.13, 115.57, 69.14, 62.97, 62.37, 60.10, 53.35, 51.91, 51.25, 49.14, 46.35, 40.84, 36.20, 31.14, 27.31, 22.73, 17.19 |
| 6m | 7.13—7.02(m, 3H), 4.48—4.43(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.83(dd, J1=14.6 Hz, J2= 4.2 Hz, 1H), 1.30—1.28(m, 6H) | 155.74, 146.18, 137.53, 131.42, 128.27, 125.68, 109.95, 63.09, 58.58, 55.75, 52.85, 50.63, 48.04, 46.12, 41.00, 36.17, 35.86, 17.06, 11.62 |
Table 4 1H NMR and 13C NMR data of compounds 6a—6m
| Compd. | 1H NMR(300 MHz), δ* | 13C NMR(75 MHz), δ* |
|---|---|---|
| 6a | 12.55(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.04(m, 1H), 4.62(t, J=5.0 Hz, 2H), 3.86—3.47(m, 4H), 3.28—3.02(m, 8H), 2.89—2.86(dd, J1=15.7Hz, J2=4.6 Hz, 1H), 1.47—1.37(m, 6H), 1.32—1.29(m, 3H) | 154.91, 145.40, 136.95, 131.18, 127.77, 126.38, 115.18, 59.16, 51.72, 49.69, 47.32, 46.34, 40.57, 35.11, 17.09, 8.32 |
| 6b | 12.66(bs, 1H), 7.13—7.11(m, 2H), 7.04—7.01(m, 1H), 4.62—4.55(m, 2H), 3.82—3.45(m, 4H), 3.33—3.19(m, 2H), 3.14—3.01(m, 2H), 2.96—2.75(m, 7H), 1.30(d, J=7.0 Hz, 3H) | 154.37, 145.07, 136.17, 132.42, 130.70, 128.18, 125.23, 58.94, 55.51, 49.64, 45.39, 42.86, 39.52, 34.88, 17.59 |
| 6c | 12.45(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.03(m, 1H), 4.65—4.64(m, 2H), 3.82—3.45(m, 6H), 3.24—3.22(m, 2H), 3.18—3.00(m, 2H), 2.88—2.83(m, 1H), 2.70—2.63(m, 2H), 2.33—2.30(m, 2H), 1.95—1.85(m, 3H), 1.50—1.37(m, 1H), 1.33—1.29(m, 3H) | 155.25, 145.55, 136.66, 132.35, 131.76, 127.85, 125.73, 59.43, 56.01, 53.48, 51.44, 46.30, 40.03, 34.97, 22.25, 21.44, 17.20 |
| 6d | 13.39(s, 1H), 7.15—7.11(m, 2H), 7.06—7.02(m, 1H), 4.64—4.40(m, 2H), 4.36—4.28(m, 2H), 4.01—3.98(m, 2H ), 3.81—3.50(m, 4H), 3.48—3.39(m, 2H), 3.30—3.22(m, 2H), 3.17—3.03(m, 2H), 2.99—2.89(m, 3H), 1.31—1.28(m, 3H) | 154.87, 145.75, 137.16, 132.16, 131.47, 128.06, 126.38, 63.92, 58.78, 56.01, 51.69, 50.95, 45.81, 39.95, 34.80, 17.11 |
| 6e | 7.15(d,J=1.9 Hz, 1H), 7.07(dd, J1=8.1 Hz, J2=1.9 Hz, 1H), 7.02(d, J=8.1 Hz, 1H), 4.19—4.17(m, 2H), 3.60—3.55(m, 4H), 3.44—3.24(m, 10H ), 3.12—3.06(m, 2H), 2.92(s, 3H), 2.79—2.74(m, 1H), 1.12(d, J=7.1 Hz, 3H) | 156.37, 145.13, 137.06, 131.19, 127.85, 126.14, 114.72, 59.43, 56.01, 50.96, 49.33, 48.83, 45.48, 42.55, 39.12, 33.26, 16.38 |
| 6f | 9.01(bs, 2H), 7.14—7.11(m, 2H), 7.07—7.03(m, 1H), 5.26—5.20(m, 1H), 3.84—3.48(m, 4H), 3.44—3.30(m, 2H), 3.12—3.00(m, 3H), 2.89(d, J=6.6 Hz, 3H),2.84(d, J=4.0 Hz, 3H), 1.31—1.29(m, 3H), 1.16(d, J=6.1 Hz, 3H) | 162.83, 154.43, 145.13, 136.66, 132.41, 127.84, 126.15, 68.73, 65.31, 60.25, 50.95, 46.29, 45.48, 42.55, 35.78, 22.25, 16.78 |
| 6g | 12.18(bs, 2H),7.16—7.12(m, 2H), 7.08—7.03(m, 1H),5.27—5.21(m, 1H), 3.78—3.44(m, 4H), 3.41—3.23(m, 6H), 3.07—2.93(m, 3H), 1.36—1.27(m, 12H) | 155.65, 145.96, 137.96, 132.01, 127.86, 126.14, 115.13, 72.97, 70.37, 58.12, 50.95, 47.60, 45.49, 40.44, 36.19, 18.91,17.35 11.73 |
| 6h | 7.11(m, 1H),7.05—6.98(m, 2H), 4.19—4.17(m, 2H), 3.60—3.57(m, 1H), 3.41—3.38(m, 6H), 3.25—3.15(m, 7H), 3.08—3.02(m, 1H), 2.96—2.88(m, 1H), 2.77—2.69(m, 1H), 1.10(d, J=7.1 Hz, 3H) | 145.95, 137.06, 132.71, 131.99, 127.84, 126.13, 114.72, 59.84, 56.00, 50.54, 48.43, 45.08, 41.66, 38.31, 33.25, 15.97. |
| 6i | 10.25(bs, 2H), 7.64—7.62(m, 2H), 7.43—7.41(m, 3H), 7.15—7.09(m, 2H), 7.04—7.01(m, 1H), 4.50—4.69(m, 2H), 4.19(s, 2H), 3.83—3.80(m, 1H), 3.60—3.55(m, 3H), 3.11—3.02(m, 4H), 2.91—2.79(m, 1H), 1.30—1.25(m, 3H) | 155.51, 145.80, 136.60, 131.30, 129.78, 129.41, 129.15, 128.79, 127.50, 125.82, 125.73, 59.90, 51.05, 49.99, 46.18, 44.92, 40.39, 35.47,17.17 |
| 6j | 11.49(bs, 0.5H), 10.83(bs, 0.5H), 9.11(bs, 0.5H), 8.79(bs, 0.5H), 7.15—7.11(m, 2H), 7.07—7.03(m, 1H), 4.60—4.37(m, 3H), 3.76—3.34(m, 7H), 3.21—3.01(m, 3H), 2.83(s, 3H), 2.72—2.61(m, 1H), 2.40—2.09(m, 3H), 1.99—1.81(m, 2H), 1.75—1.59(m, 1H), 1.36—1.30(m, 3H) | 175.06, 155.65, 145.13, 137.96, 132.00, 128.66, 126.13, 114.72, 67.84, 62.77, 55.20, 51.85, 51.30, 46.79, 41.38, 40.84, 36.60, 29.02, 25.68, 24.78, 23.15, 17.61 |
| 6k | 7.15—7.12(m, 1H), 7.09—7.01(m, 2H), 4.19—4.14(m, 2H), 3.69—3.63(m, 2H ), 3.53—3.36(m, 5H), 3.23—3.02(m, 3H), 3.02—2.88(m, 1H), 2.85—2.71(m, 1H), 2.63(d, J =5.6 Hz, 3H), 2.51—2.32(m, 1H), 2.09—1.93(m, 3H), 1.12(d, J=6.5 Hz, 3H) | 167.89, 156.56, 146.36, 137.06, 132.00, 127.85, 126.55, 115.13, 67.42, 64.49, 60.25, 56.50, 50.13, 46.31, 39.14, 32.85, 30.15, 29.42, 26.49, 22.25, 16.38 |
| 6l | 7.13—7.09(m, 2H), 7.05—7.02(m, 1H), 4.50—4.18(m, 2H), 3.91—3.30(m, 10H), 3.23—3.01(m, 3H), 2.86—2.81(m, 1H), 2.09—1.82(m, 8H), 1.33—1.25(m, 3H) | 156.06, 146.36, 137.97, 132.41, 128.25, 126.13, 115.57, 69.14, 62.97, 62.37, 60.10, 53.35, 51.91, 51.25, 49.14, 46.35, 40.84, 36.20, 31.14, 27.31, 22.73, 17.19 |
| 6m | 7.13—7.02(m, 3H), 4.48—4.43(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.83(dd, J1=14.6 Hz, J2= 4.2 Hz, 1H), 1.30—1.28(m, 6H) | 155.74, 146.18, 137.53, 131.42, 128.27, 125.68, 109.95, 63.09, 58.58, 55.75, 52.85, 50.63, 48.04, 46.12, 41.00, 36.17, 35.86, 17.06, 11.62 |
| Compd. | Metabolic results | ||||
|---|---|---|---|---|---|
| Regression equation (1) | Half-intial concentration/ (μmol·L-1) | Regression equation (2) | |||
| Lorcaserin | y=0.0625x-0.1457 | 0.9806 | 7.958 | y=0.0028x+0.048 | 0.9724 |
| 6a | y=0.0486x-0.0756 | 0.9850 | 9.946 | y=0.0014x+0.0571 | 0.9774 |
| 6b | y=0.0607x-0.1028 | 0.9945 | 9.068 | y=0.003x+0.0415 | 0.9788 |
| 6c | y=0.0725x+0.1009 | 0.9719 | 7.477 | y=0.0725x+0.1009 | 0.9402 |
| 6d | y=0.0527x-0.0604 | 0.9896 | 8.429 | y=0.0032x+0.0874 | 0.9595 |
| 6e | y=0.0757x+0.0892 | 0.9889 | 10.349 | y=0.0009x+0.0664 | 0.9634 |
| 6f | y=0.0572x-0.1324 | 0.9813 | 8.457 | y=0.0016x+0.0546 | 0.9935 |
| 6g | y=0.0451x-0.0776 | 0.9973 | 9.297 | y=0.0115x+0.0138 | 0.9411 |
| 6h | y=0.0561x+0.2282 | 0.9960 | 12.253 | y=0.047x-0.3031 | 0.9393 |
| 6i | y=0.0585x-0.0448 | 0.9874 | 8.939 | y=0.0046x+0.0421 | 0.9655 |
| 6j | y=0.0535x-0.0584 | 0.9994 | 9.892 | y=0.0035x+0.0724 | 0.9165 |
| 6k | y=0.0500x-0.0300 | 0.9988 | 14.680 | y=0.0049x+0.0038 | 0.9474 |
| 6l | y=0.0623x+0.0208 | 0.9962 | 10.443 | y=0.0083x-0.0347 | 0.9384 |
| 6m | y=0.0663x+0.2536 | 0.9862 | 9.392 | y=24.877 | 0.9806 |
Table 5 Metabolic results of compounds 6a—6m
| Compd. | Metabolic results | ||||
|---|---|---|---|---|---|
| Regression equation (1) | Half-intial concentration/ (μmol·L-1) | Regression equation (2) | |||
| Lorcaserin | y=0.0625x-0.1457 | 0.9806 | 7.958 | y=0.0028x+0.048 | 0.9724 |
| 6a | y=0.0486x-0.0756 | 0.9850 | 9.946 | y=0.0014x+0.0571 | 0.9774 |
| 6b | y=0.0607x-0.1028 | 0.9945 | 9.068 | y=0.003x+0.0415 | 0.9788 |
| 6c | y=0.0725x+0.1009 | 0.9719 | 7.477 | y=0.0725x+0.1009 | 0.9402 |
| 6d | y=0.0527x-0.0604 | 0.9896 | 8.429 | y=0.0032x+0.0874 | 0.9595 |
| 6e | y=0.0757x+0.0892 | 0.9889 | 10.349 | y=0.0009x+0.0664 | 0.9634 |
| 6f | y=0.0572x-0.1324 | 0.9813 | 8.457 | y=0.0016x+0.0546 | 0.9935 |
| 6g | y=0.0451x-0.0776 | 0.9973 | 9.297 | y=0.0115x+0.0138 | 0.9411 |
| 6h | y=0.0561x+0.2282 | 0.9960 | 12.253 | y=0.047x-0.3031 | 0.9393 |
| 6i | y=0.0585x-0.0448 | 0.9874 | 8.939 | y=0.0046x+0.0421 | 0.9655 |
| 6j | y=0.0535x-0.0584 | 0.9994 | 9.892 | y=0.0035x+0.0724 | 0.9165 |
| 6k | y=0.0500x-0.0300 | 0.9988 | 14.680 | y=0.0049x+0.0038 | 0.9474 |
| 6l | y=0.0623x+0.0208 | 0.9962 | 10.443 | y=0.0083x-0.0347 | 0.9384 |
| 6m | y=0.0663x+0.2536 | 0.9862 | 9.392 | y=24.877 | 0.9806 |
| Group | Animal number | Weight/g | |
|---|---|---|---|
| Before experimenta | After experimentb | ||
| Normal diet control | 6 | 222.33±9.29 | 322.17±7.96 |
| High fat control | 6 | 230.83±5.11 | 502.50±22.83** |
| Lorcaserin control | 6 | 228.67±6.00 | 470.17±15.17** |
| Compound 6b | 6 | 228.33±8.56 | 496.00±20.67** |
Table 6 Effect of fodder on body-weigh in model rats(x?±s, n=6)
| Group | Animal number | Weight/g | |
|---|---|---|---|
| Before experimenta | After experimentb | ||
| Normal diet control | 6 | 222.33±9.29 | 322.17±7.96 |
| High fat control | 6 | 230.83±5.11 | 502.50±22.83** |
| Lorcaserin control | 6 | 228.67±6.00 | 470.17±15.17** |
| Compound 6b | 6 | 228.33±8.56 | 496.00±20.67** |
| Compd. | Half-life period/min | Compd. | Half-life period/min | Compd. | Half-life period/min |
|---|---|---|---|---|---|
| Lorcaserin | 27.736 | 6e | 33.586 | 6j | 8.198 |
| 6a | 31.031 | 6f | 39.778 | 6k | 13.127 |
| 6b | 22.926 | 6g | 8.153 | 6l | 15.718 |
| 6c | 14.754 | 6h | 8.185 | 6m | 12.488 |
| 6d | 9.762 | 6i | 15.167 |
Table 7 Half-life period of compounds 6a—6m
| Compd. | Half-life period/min | Compd. | Half-life period/min | Compd. | Half-life period/min |
|---|---|---|---|---|---|
| Lorcaserin | 27.736 | 6e | 33.586 | 6j | 8.198 |
| 6a | 31.031 | 6f | 39.778 | 6k | 13.127 |
| 6b | 22.926 | 6g | 8.153 | 6l | 15.718 |
| 6c | 14.754 | 6h | 8.185 | 6m | 12.488 |
| 6d | 9.762 | 6i | 15.167 |
| Group | Animal number | Weight/g | Weight increase/g | |
|---|---|---|---|---|
| Before experimenta | After experimentb | |||
| Normal diet control | 6 | 322.17±7.96 | 325.00±15.43 | 2.83±13.54 |
| High fat control | 6 | 502.50±22.83** | 550.33±19.00** | 47.83±15.89** |
| Lorcaserin control | 6 | 470.17±15.17** | 481.83±18.22**## | 11.67±11.67# |
| Compound 6b | 6 | 496.00±20.67** | 490.83±28.50**## | -5.17±17.28## |
Table 8 Effect of compound 6b on body-weigh in high-fat model rats(xˉ±s, n=6)
| Group | Animal number | Weight/g | Weight increase/g | |
|---|---|---|---|---|
| Before experimenta | After experimentb | |||
| Normal diet control | 6 | 322.17±7.96 | 325.00±15.43 | 2.83±13.54 |
| High fat control | 6 | 502.50±22.83** | 550.33±19.00** | 47.83±15.89** |
| Lorcaserin control | 6 | 470.17±15.17** | 481.83±18.22**## | 11.67±11.67# |
| Compound 6b | 6 | 496.00±20.67** | 490.83±28.50**## | -5.17±17.28## |
| Group | Animal number | Liver index | Lipid index | Lees index |
|---|---|---|---|---|
| Normal diet control | 6 | 2.95±0.53 | 0.85±0.33 | 290.66±5.05 |
| High fat control | 6 | 3.89±0.27** | 3.04±0.53** | 311.77±4.18** |
| Lorcaserin control | 6 | 3.97±0.25** | 2.70±0.68** | 306.66±4.08** |
| Compound 6b | 6 | 3.61±0.34* | 2.09±0.48** | 304.45±5.20** |
Table 9 Effect of new compound in high-fat model rats(xˉ±s, n=6)
| Group | Animal number | Liver index | Lipid index | Lees index |
|---|---|---|---|---|
| Normal diet control | 6 | 2.95±0.53 | 0.85±0.33 | 290.66±5.05 |
| High fat control | 6 | 3.89±0.27** | 3.04±0.53** | 311.77±4.18** |
| Lorcaserin control | 6 | 3.97±0.25** | 2.70±0.68** | 306.66±4.08** |
| Compound 6b | 6 | 3.61±0.34* | 2.09±0.48** | 304.45±5.20** |
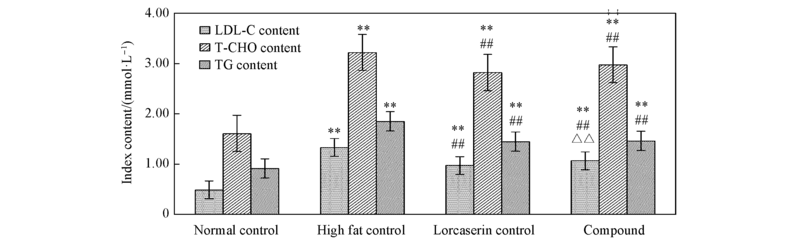
Fig.3 Effect of compound 6b on blood serum T-CHO, TG, LDL-C in model rats**P<0.01 compared with the normal diet control group; ## P<0.01 compared with the high fat control group; △△ P<0.01 compared with the compound control group.
| [1] | Lam D. D., Przydzial M. J., Ridley S. H., Yeo G. S., Rochford J. J., O’Rahilly S., Heisler L. K., Endocrinology,2008, 149(3), 1323—1328 |
| [2] | Ioannides-Demos L. L., Proietto J., Tonkin A. M., McNeil J. J., Drug Safety,2006, 29(4), 277—302 |
| [3] | Kim G. W., Lin J. E., Blomain E. S., Waldman S. A., Expert Opin. Drug Discov., 2013, 8(6), 655—671 |
| [4] | Topol E. J., Bousser M. G., Fox K. A., Creager M. A., Despres J. P., Easton J. D., Hamm C. W., Montalescot G., Steg P. G., Pearson T. A., Cohen E., Gaudin C., Job B., Murphy J. H., Bhatt D. L., Lancet,2010, 376(9740), 517—523 |
| [5] | Chaudhari D., Crisostomo C., Ganote C., Youngberg G., Case Rep. Nephrol., 2013, 2013, 124604 |
| [6] | Khazaal Y., Zullino D. F., Eur. J. Clin. Pharmacol., 2007, 63(9), 891—892 |
| [7] | Gadde K. M., Parker C. B., Maner L. G., Wagner H. R., Logue E. J., Drezner M. K., Krishnan K. R., Obes. Res., 2001, 9(9), 544—551 |
| [8] | Plodkowski R. A., Nguyen Q., Sundaram U., Nguyen L., Chau D. L., St Jeor S., Expert Opin. Pharmacother., 2009, 10(6), 1069—1081 |
| [9] | Waser B., Beetschen K., Pellegata N. S., Reubi J. C., Neuroendocrinology,2011, 94(4), 291—301 |
| [10] | Wang Y., Zheng Q. C., Chem. J. Chinese Universities,2015, 36(11), 2226—2235 |
| (王衍, 郑清川. 高等学校化学学报, 2015, 36(11), 2226—2235) | |
| [11] | Wang Y., Zheng Q. C., Zhang J. L., Xie M., Zhan J. Y., Zhang H. X., Chem. Res. Chinese Universities,2015, 31(6), 1029—1038 |
| [12] | Usmani K. A., Chen W. G., Sadeque A. J., Drug Metab. Dispos., 2012, 40(4), 761—771 |
| [13] | Arena Pharmaceuticals, Belviq(Lorcaserin Hydrochloride) Prescribing Information., 2012, 2012-06-29, |
| [14] | Jr S. J. D., FotschC., J. Med. Chem., 2012, 55(13), 6002—6020 |
| [15] | Sadeque A. J., Usmani K. A., Palamar S., Cerny M. A., Chen W. G., Drug Metab. Dispos., 2012, 40(4), 772—778 |
| [16] | Wang X. Y., Ye J., Sun G. X., Xue B. J., Zhao Y. Y., Miao P. P., Su J., Zhang Y. J., Chinese Journal of Chinese Materia Medica,2014, 39(19), 3855—3859 |
| (杨晓燕, 叶静, 孙桂霞, 薛宝娟, 赵圆圆, 苗培培, 苏瑾, 张玉杰. 中国中药杂志, 2014, 39(19), 3855—3859) | |
| [17] | Usmani K. A., Chen W. G., Sadeque A. J., Drug Metab. Dispos., 2012, 40(4), 761—771 |
| [18] | Thomsen W. J., Grottick A. J., Menzaghi F., Reyes-Saldana H., Espitia S., Yuskin D., Whelan K., Martin M., Morgan M., Chen W., Al-Shamma H., Smith B., Chalmers D., Behan D., J. Pharmacol. Exp. Ther., 2008, 325(2), 577—587 |
| [19] | Smith B. M., Smith J. M., Tsai J. H., Schultz J. A., Gilson C. A., Estrada S. A., Chen R. R., Park D. M., Prieto E. B., Gallardo C. S., Sengupta D., Dosa P. I., Covel J. A., Ren A., Webb R. R., Beeley N. R., Martin M., Morgan M., Espitia S., Saldana H. R., Bjenning C., Whelan K. T., Grottick A. J., Menzaghi F., Thomsen W. J., J. Med. Chem., 2008, 51(2), 305—313 |
| [20] | Tian H., Wang Y. T., Tao L., Ding H., Chinese Pharmacological Bulletin,2013, 29(7), 1016—1024 |
| (田辉, 王玉婷, 陶莉, 丁虹. 中国药理学通报, 2013, 29(7), 1016—1024) |
| [1] | REN Yushuang, GUO Yuanyuan, LIU Xueyi, SONG Jie, ZHANG Chuan. Platinum(Ⅳ) Prodrug-grafted Phosphorothioate DNA and Its Self-assembled Nanostructure for Targeted Drug Delivery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1721. |
| [2] | WANG Cheng-Yun, GONG Yan-Bao, LIU Shun-Ying, LUO Shu-Fang, HUANG Jing, YU Jia-Hui*. Synthesis of α,β-Poly[(N-succinyl)-L-aspartamide] and in vitro Cytotoxicity of Its cis-Dichlorodiammine Platinum Macromolecular Prodrug [J]. Chem. J. Chinese Universities, 2008, 29(8): 1665. |
| [3] | WANG Yin-Song1*, HAN Yue-Lian2, LI Ying-Xia3, WANG Yu-Mei1, LI Rong-Shan1. Preparation and in vitro Experimental Study of Methotrexate-Lactosyl-Chitosan Conjugate [J]. Chem. J. Chinese Universities, 2007, 28(6): 1092. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||