

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (12): 2448.doi: 10.7503/cjcu20190352
• Articles: Inorganic Chemistry • Previous Articles Next Articles
Hongjie WANG2,Chongyang ZHU1,2,Su ZHANG1,*( ),Ran PANG1,Lihong JIANG1,Da LI1,Guanyu LIU1,Jize CAI1,Jing FENG1,Chengyu LI1,*(
),Ran PANG1,Lihong JIANG1,Da LI1,Guanyu LIU1,Jize CAI1,Jing FENG1,Chengyu LI1,*( )
)
Received:2019-06-24
Online:2019-12-04
Published:2019-12-04
Contact:
Su ZHANG,Chengyu LI
E-mail:zhangsu@ciac.ac.cn;cyli@ciac.ac.cn
Supported by:TrendMD:
Hongjie WANG,Chongyang ZHU,Su ZHANG,Ran PANG,Lihong JIANG,Da LI,Guanyu LIU,Jize CAI,Jing FENG,Chengyu LI. Structure and Host Luminescence of YXO4(X=Nb, V) †[J]. Chem. J. Chinese Universities, 2019, 40(12): 2448.
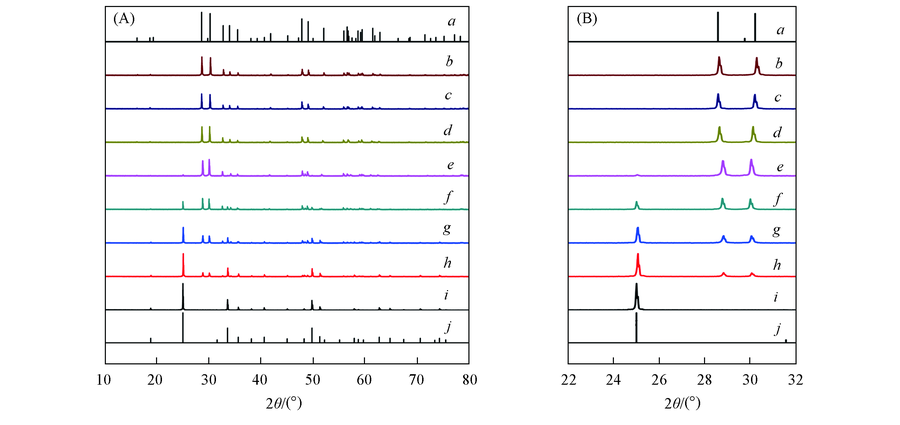
Fig.2 XRD patterns(A) and part enlarged patterns(B) of samples a. JCPDS No.23-1486; b. YNbO4; c. YNb0.98O4:0.02V; d. YNb0.92O4:0.08V; e. YNb0.8O4:0.2V;f. YNb0.6O4:0.4V; g. YNb0.4O4:0.6V; h. YNb0.2O4:0.8V; i. YVO4; j. JCPDS No.17-0341.
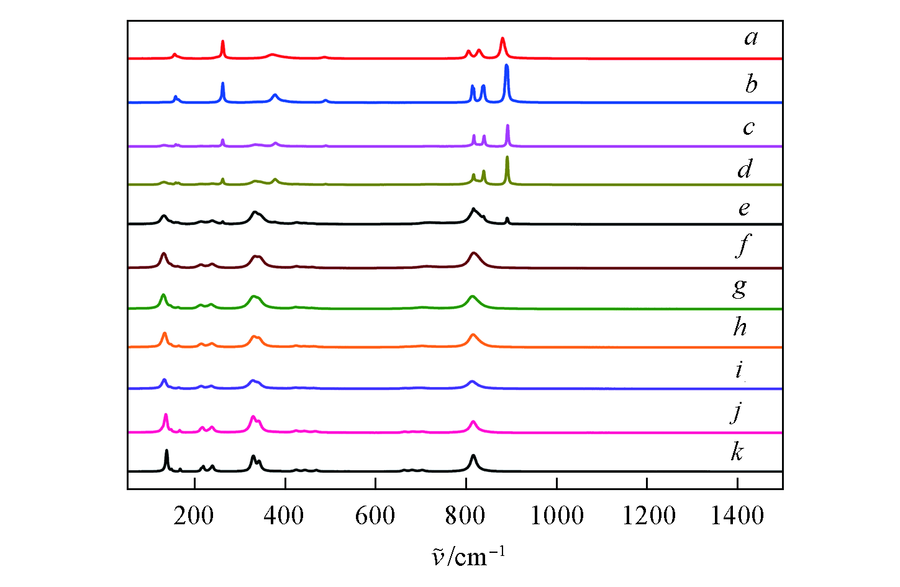
Fig.4 Raman spectra of YNb1-xO4:xV samples at room temperature a. YVO4; b. YNb0.2O4:0.8V; c. YNb0.4O4:0.6V; d. YNb0.6O4:0.4V; e. YNb0.8O4:0.2V; f. YNb0.85O4:0.15V; g. YNb0.9O4:0.1V; h. YN b 0.9 2 O4:0.08V; i. YN b 0.9 6 O4:0.04V; j. YNb0.98O4:0.02V; k. YNbO4.
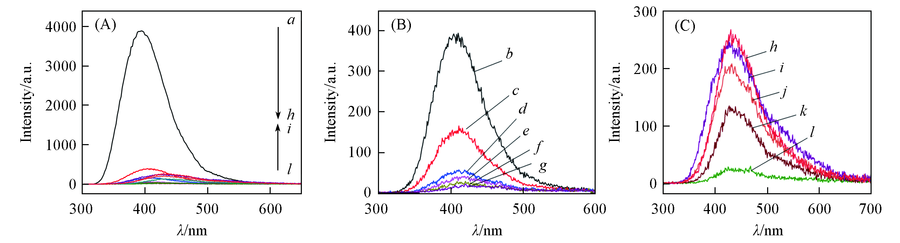
Fig.5 Emission spectra of YNb1-xO4:xV excited by 257 nm ultraviolet light at room temperature x: a. 0; b. 0.01; c. 0.02; d. 0.04; e. 0.08; f. 0.1; g. 0.15; h. 0.2; i. 0.4; j. 0.6; k. 0.8; l. 1.
| [1] | Li L. W., Lv Y., Niu C. H., Lang X. P ., Chinese J. Lumin., 2018,39, 449— 456 |
| ( 李磊伟, 吕勇, 牛春晖, 郎晓萍 . 发光学报, 2018,39, 449— 456) | |
| [2] |
Loiko P. A., Dymshits O. S., Alekseeva I. P., Zhilin A. A., Tsenter M. Y., Vilejshikova E. V., Bogdanov K. V., Mateos X., Yumashev K. V ., J. Lumin., 2016,179, 64— 73
doi: 10.1016/j.jlumin.2016.06.042 URL |
| [3] |
Nakajima T., Isobe M., Tsuchiya T., Ueda Y., Kumagai T ., J. Lumin., 2009,129, 1598— 1601
doi: 10.1016/j.jlumin.2009.03.029 URL |
| [4] | Yang M ., Niobate: High Temperature and High Pressure Synthesis and Fluorescence Properties, Jilin University, Changchun, 2013 |
| ( 杨敏 . 铌酸盐:高温高压合成与荧光性质的研究, 长春: 吉林大学, 2013) | |
| [5] |
Blasse G., Bril A ., J. Lumin., 1970,3, 109— 131
doi: 10.1016/0022-2313(70)90011-6 URL |
| [6] |
Brixner L. H., Chen H. Y ., J. Electrochem. Soc., 1983,130(12), 2 435— 2443
doi: 10.1149/1.2119609 URL |
| [7] |
Lammes M., Blasse G ., Mater. Res. Bull., 1984,19, 759— 768
doi: 10.1016/0025-5408(84)90033-3 URL |
| [8] | Buth A. H., Blasse G ., Phys. Status Solidi A, 1981,64(2), 669— 676 |
| [9] |
Blasse G ., J. Solid State Chem., 1973,7, 169— 171
doi: 10.1016/0022-4596(73)90151-5 URL |
| [10] | Yang W. Q ., J. Lumin., 2001,22(2), 175— 181 |
| ( 杨文琴 . 发光学报, 2001,22(2), 175— 181) | |
| [11] | Lan J. M., Chen J. Z., Guo F. Y., Gao S. K, . Hu X. L., Zhuang N. F ., Journal of Synthetic Crystals, 2003,32(1), 44— 49 |
| ( 兰建明, 陈建中, 郭飞云, 高绍康, 胡晓琳, 庄乃锋 . 人工晶体学报, 2003,32(1), 44— 49) | |
| [12] |
Chen X. B., Zhou G., Zhou Y. F., Wu Z. L., Guo Y. Y., Wang S. F., Zhou Q. Y., Zhuang J., Chen X. D., Li C. M., Yao W. T., Cheng H. L ., Spectroscopy and Spectral Analysis, 2015,35(2), 315— 319
URL pmid: 25970884 |
|
( 陈晓波, 周固, 周永芬, 吴正龙, 郭玉英, 王水锋, 邹秋燕, 庄建, 陈晓端, 李春密, 姚文婷, 程欢利 . 光谱学与光谱分析, 2015,35(2), 315— 319)
URL pmid: 25970884 |
|
| [13] | Wang Z. Z., Shen L. J., Li B., Gao L. L., Zhang Y. B., Zhao J., Zhang G. B ., Chinese Journal of Rare Earth, 2013,31(4), 431— 435 |
| ( 王忠志, 沈雷军, 李波, 高乐乐, 周永勃, 赵静, 张国斌 . 中国稀土学报, 2013,31(4), 431— 435) | |
| [14] |
Kang F. W., Yang X. B., Peng M. Y., Wondraczek L., Ma Z. J., Zhang Q. Y., Qiu J. R ., J. Phys. Chem. C, 2014,118(14), 7515— 7522
doi: 10.1021/jp4081965 URL |
| [15] |
Tian Y Y., Tian Y., Huang P., Wang L., Shi Q. F., Cui C. E ., Chem. Eng. J., 2016,297, 26— 34
doi: 10.1016/j.cej.2016.03.149 URL |
| [16] |
Yang M., Zhao X D., Ji Y., Liu F Y., Liu W., Sun J Y., Liu X Y ., New J. Chem., 2014,38, 4249— 4257
doi: 10.1039/C4NJ00399C URL |
| [17] |
Arai M., Wang Y. X., Kohiki S., Matsuo M., Shimooka H., Shishido T., Oku M ., Jpn. J. Appl. Phys., 2005,44, 6596— 6599
doi: 10.1143/JJAP.44.6596 URL |
| [18] |
Kim D. W., Kwon D.K., Yoon S. H., Hong K. S ., J. Am. Ceram. Soc., 2006,89, 3861— 3864
doi: 10.1111/jace.2006.89.issue-12 URL |
| [19] |
Haugsrud R., Norby T ., Nat. Mater., 2006,5, 193— 196
doi: 10.1038/nmat1591 URL |
| [20] |
Haugsrud R., Norby T ., Solid State Ionics, 2006,177, 1129— 1135
doi: 10.1016/j.ssi.2006.05.011 URL |
| [21] |
Zhang S., Lv L., Wang H., Zhu C., Pang R., Feng J., Li D., Liu G., Jiang L., Li C ., J. Lumin., 2019,211, 183— 192
doi: 10.1016/j.jlumin.2019.03.036 URL |
| [22] |
Pradhan A. K., Choudhary R. N. P ., Phys. Status Solidi B, 1987,143, K161— K166
doi: 10.1002/(ISSN)1521-3951 URL |
| [23] |
Blasse G ., J. Solid State Chem., 1973,7, 169— 171
doi: 10.1016/0022-4596(73)90151-5 URL |
| [24] | Kawakami S., Takeda N., Kohiki S., Tsutsui F., Harada J., Arai M., Mitome M., Ohmura K., Yubuta K., Shishido T ., Emerg. Mater. Res., 2013,2, 191— 197 |
| [25] | Chen Y. Z., Li S., Li L., Men Z. W., Li Z. L., Sun C. L., Li Z. W., Zhou M ., J. Physics, 2013,62, 243— 247 |
| ( 陈元正, 李硕, 李亮, 门志伟, 李占龙, 孙成林, 里佐威, 周密 . 物理学报, 2013,62, 243— 247) | |
| [26] | Dahl J. P., Ballhausen C.J ., Adv. Quantum Chem., 1968,4, 170— 226 |
| [27] |
Dahl J. P., Johansen H ., Theor. Chim. Acta, 1968,11, 8— 25
doi: 10.1007/BF00526064 URL |
| [28] |
Aia M. A , J. Electrochem. Soc., 1967,114, 367— 370
doi: 10.1149/1.2426598 URL |
| [29] | Buth A. H., Blasse G ., Phys. Status Solidi A, 1981,64, 669— 676 |
| [30] |
Weitzel H., Schröcke H ., Z. Kristallogr., 1980,152, 69— 82
doi: 10.1524/zkri.1980.152.1-2.69 URL |
| [31] |
Blasse G ., J. Solid State Chem., 1973,7, 169— 171
doi: 10.1016/0022-4596(73)90151-5 URL |
| [32] |
Lee S. K., Chang H., Han C. H., Kim H. J., Jang H. G., Park H. D ., J. Solid State Chem., 2001,156, 267— 273
doi: 10.1006/jssc.2000.8941 URL |
| [1] | ZHANG Min, CHEN Mengwei, GAO Hong, BI Yanfeng. Synthesis, Structure and Electrochemical Properties of Sulfonylcalix[4]arene Supported Co16 Cluster † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2052. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||