

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (5): 897.doi: 10.7503/cjcu20170672
Previous Articles Next Articles
HUANG Chibao*, PAN Qi, CHEN Huashi, LIANG Xing, LÜ Guoling
Received:2017-10-11
Online:2018-04-08
Published:2018-04-08
Contact:
HUANG Chibao
Supported by:CLC Number:
TrendMD:
HUANG Chibao,PAN Qi,CHEN Huashi,LIANG Xing,LÜ Guoling. Dicyanostilbene-derived Two-photon Fluorescence Probe for Lead Ions†[J]. Chem. J. Chinese Universities, 2018, 39(5): 897.
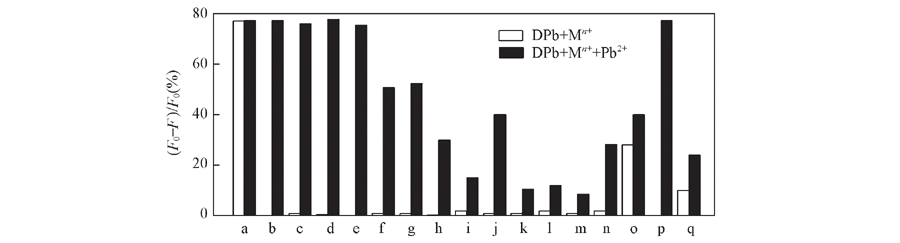
Fig.2 Relative fluorescence intensity of 1 μmol/L DPb in the presence of 20 mmol/L Pb2+ and other ions(empty bars) followed by addition of 40 μmol/L Pb2+(filled bars) Note:a—q: Pb2+, K+, Ca2+, Mg2+, Ba2+, Fe2+, Fe3+, Zn2+, Co2+, Ni2+, Cr3+, Cd2+, Mn2+, Cu2+, Ag+, Na+, Hg2+. Data were measured in 30 mmol/L MOPS buffer(100 mmol/L KCl+10 mmol/L EGTA, pH=7.2). λex1=400 nm, λex2=790 nm.
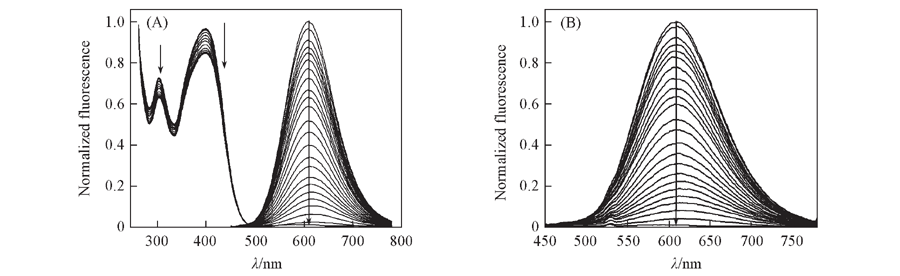
Fig.3 Absorption(A) and emission spectra(B) of DPb(1 μmol/L) in 30 mmol/L MOPS buffer(100 mmol/L KCl+10 mmol/L EGTA, pH=7.2) upon the addition of Pb2+(0—40 μmol/L), respectively Note:λex1=400 nm, λex2=790 nm. (A) Left: absorption spectra; right: one-photon emission spectra; (B) two-photon emission spectra.
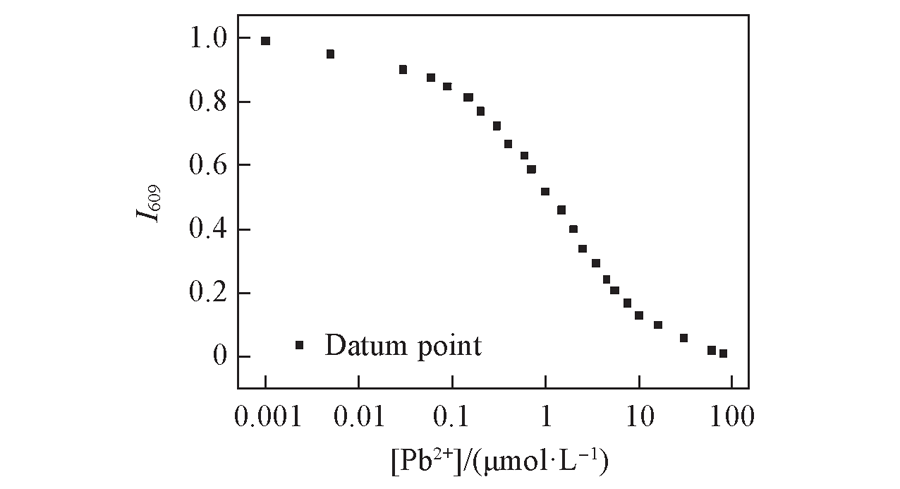
Fig.4 Best fitting curve between changes in one-photon absorption intensities of DPb(10 μmol/L) at 400 nm and Pb2+ concentrations Note:Data were measured in 30 mmol/L MOPS buffer (100 mmol/L KCl+10 mmol/L EGTA, pH=7.2).
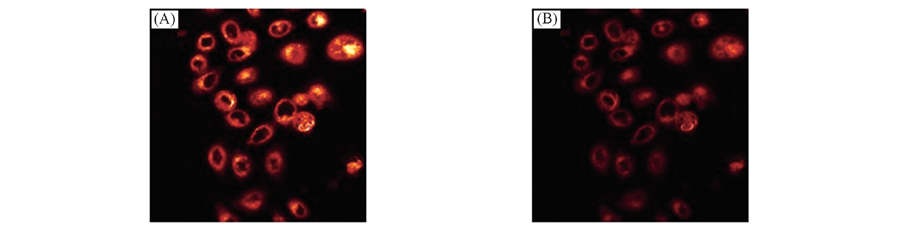
Fig.5 TPM images of 1 μmol/L DPb-labeled mouse fibroblast collected at 550—650 nm before(A) and after(B) the addition of 20 μmol/L Pb2+ to the imaging solution Note: The two-photon excitation fluorescence(TPEF) images were collected upon excitation at 790 nm with a femtosecond pulse. Cells shown are representative images from replicate experiments(n=5).
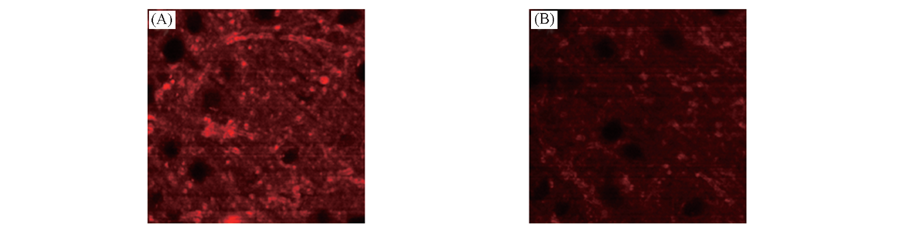
Fig.6 TPM images of a mouse brain tissue slice stained with 10 μmol/L DPb at a depth of ca. 120 μm with magnification 100× before(A) and after(B) the addition of 20 μmol/L Pb2+ to the imaging solution Note:The two-photon excitation fluorescence(TPEF) images were collected at 550—650 nm upon excitation at 790 nm with a femtosecond pulse. Cells shown are representative images from replicate experiments(n=5).
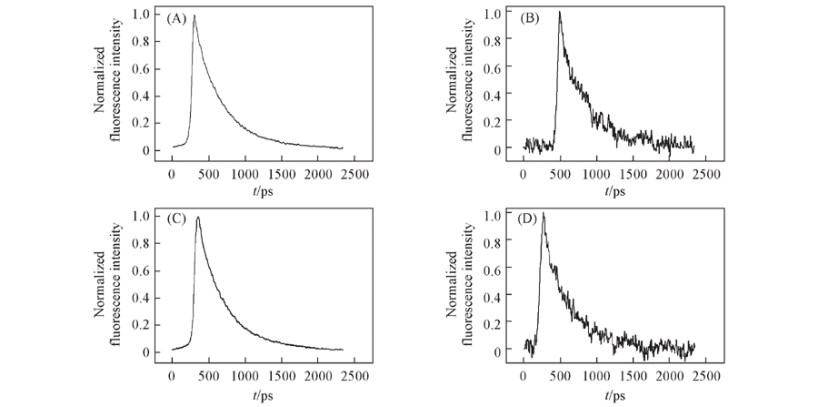
Fig.7 Normalized fluorescence decay curves of DPb(10 μmol/L) solutions at 609 nm before and after the addition of Pb2+ excited by one-photon absorption(403 nm) and two-photon absorption(790 nm) Note:(A) OPFL(DPb-Pb2+); (B) TPFL(DPb-Pb2+); (C) OPFL(DPb); (D) TPFL(DPb). OPFL and TPFL abbreviated from one- and two-photon fluorescence lifetime, respectively.
| [1] | Meyer P.A., Pivetz T., Dignam T. A., Homa D. M., Schoonover J., Brody D., Morbidity and Mortality Weekly Report, 2003, 52(10), 1—21 |
| [2] | Jedrychowski W., Perera F., Jankowski J., Rauh V., Flak E., Caldwell K.L., Jones R. L., Pac A., Lisowska-Miszczyk I., Int. [J]. Hyg. Environ. Health., 2008, 211(3/4), 345—351 |
| [3] | The European Parliament and the Council of the European Union, Directive on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment, 2011/65/EU |
| [4] | Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report, 2000, 49(5), 97—100 |
| [5] | Tahan J.E., Granadillo V. A., Romero R. A., Anal. Chim. Acta, 1994, 295(1/2), 187—197 |
| [6] | Badiei H.R., Liu C., Karanassios V., Microchem. J., 2013, 108, 131—136 |
| [7] | Li P.C., Jiang S. [J]., Anal. Bioanal. Chem., 2006, 385(6), 1092—1097 |
| [8] | Bonfil Y., Kirowa-Eisner E., Anal. Chim. Acta, 2002, 457(2), 285—296 |
| [9] | Lin S.Y., Wu S. H., Chen C. H., Angew. Chem. Int. Ed., 2006, 45(30), 4948—4951 |
| [10] | Lin Y.W., Huang C. C., Chang H. T., Analyst, 2011, 136(5), 863—871 |
| [11] | Ferhan A.R., Guo L., Zhou X., Chen P., Hong S., Kim D. H., Anal. Chem., 2013, 85(8), 4094—4099 |
| [12] | Kuo S.Y., Li H. H., Wu P. J., Chen C. P., Huang Y. C., Chan Y. H., Anal. Chem., 2015, 87(9), 4765—4771 |
| [13] | Wang X., Han B., Gao Y., Wang L., Bai M., Chem. Res. Chinese Universities, 2016, 32(3), 325—328 |
| [14] | Nie J., He B., Cheng Y.M., Yin W., Hou C. J., Huo D. Q., Qian L. L., Qin Y. N., Fa H. B., Chem. Res. Chinese Universities, 2017, 33(6), 951—957 |
| [15] | Zhang B.B., Liu X. H., Li D. N., Tian H., Ma G. P., Chang J., Chinese Science Bulletin, 2004, 52(24), 2846—2851 |
| (张兵波, 刘旭辉, 李德娜, 田惠, 马贵平, 常津. 科学通报, 2004, 52(24), 2846—2851) | |
| [16] | Yu M., He F., Tang Y., Wang S., Li Y., Zhu D., Macromol. Rapid Commun., 2007, 28(12), 1333—1338 |
| [17] | He Q., Miller E.V., Wong A. P., Chang C. J., [J]. Am. Chem. Soc., 2006, 128(29), 9316—9317 |
| [18] | Rémi M., Isabelle L., Bernard V., Chem. Eur. J., 2004, 10(18), 4480—4490 |
| [19] | Chen C.T., Huang W. P., [J]. Am. Chem. Soc., 2002, 124(22), 6246—6247 |
| [20] | Huang C.B., Chen S. Y., Progress in Chemistry, 2017, 29(10), 1216—1227 |
| (黄池宝, 陈绍英. 化学进展, 2017, 29(10), 1216—1227) | |
| [21] | Huang C.B., Yi D. S., Feng C. H., Ren A. X., Sun S. G., Progress in Chemistry, 2010, 22(12), 2408—2419 |
| (黄池宝, 易道生, 冯承浩, 任安祥, 孙世国. 化学进展, 2010, 22(12), 2408—2419) | |
| [22] | Huang C.B., Fan J. L., Peng X. J., Sun S. G., Progress in Chemistry, 2007, 19(11), 1807—1811 |
| (黄池宝, 樊江莉, 彭孝军, 孙世国. 化学进展, 2007, 19(11), 1807—1811) | |
| [23] | Cahalan M.D., Parker I., Wei S. H., Miller M. [J]., Nat. Rev. Immunol., 2002, 2(11), 872—880 |
| [24] | So P.T. C., Dong C. Y., Masters B. R., Berland K. M., Annu. Rev. Biomed. Eng., 2000, 2(1), 399—429 |
| [25] | Huang C.B., Zhang D. H., Zeng B. P., Liu Q. B., Chen H. S., Kang S., Chen X. Y., Chem. [J]. Chinese Universities, 2016, 37(4), 638—642 |
| (黄池宝, 张道海, 曾伯平, 刘其斌, 陈华仕, 康帅, 陈晓远. 高等学校化学学报, 2016, 37(4), 638—642) | |
| [26] | Diaspro A., Robello M., J. Photochem. Photobiol. B: Bio., 2000, 55(1), 1—8 |
| [27] | Köckel R.H., Cao J., Zipfel W. R., Webb W. W., Hanson M. R., Science, 1997, 276(5321), 2039—2042 |
| [28] | Denk W., Strickler J.H., Webb W. W., Science, 1990, 248(4951), 73—76 |
| [29] | Cui J.Q., Fan J. L., Peng X. J., Sun S. G., Chen G. C., Guo K. X., Science in China Series B: Chemistry, 2009, 39(6), 541—547 |
| [30] | Kim J.S., Kim H. J., Kim H. M., Kim S. H., Lee J. W., Kim S. K., Cho B. R., [J]. Org. Chem., 2006, 71(21), 8016—8022 |
| [31] | Ahn H.C., Yang S. K., Kim H. M., Li S., Jeon S. J., Cho B. R., Chem. Phys. Lett., 2005, 410(4—6), 312—315 |
| [32] | Huang C.B., Liang X., Zeng Q. H., Chen H. S., Zeng B. P., Yi D. S., Chen X. Y., Chem. [J]. Chinese Universities, 2015, 36(4), 646—653 |
| (黄池宝, 梁兴, 曾启华, 陈华仕, 曾伯平, 易道生, 陈晓远. 高等学校化学学报, 2015, 36(4), 646—653) | |
| [33] | Huang C.B., Chen H. S., Zeng B. P., Chen X. Y., Chin. [J]. Anal. Chem., 2015, 43(4), 507—511 |
| (黄池宝, 陈华仕, 曾伯平, 陈晓远. 分析化学, 2015, 43(4), 507—511) | |
| [34] | Huang C.B., Pan Q., Chen X. Y., Zhao G. L., Chen H. S., Liang X., Lü G. L., Chem. [J]. Chinese Universities, 2017, 38(10), 1751—1756 |
| (黄池宝, 潘淇, 陈晓远, 赵光练, 陈华仕, 梁兴, 吕国岭. 高等学校化学学报, 2017, 38(10), 1751—1756) | |
| [35] | Huang C.B., Ren A. X., Li H. B., Yang N. F., Chem. [J]. Chinese Universities, 2010, 31(11), 2222—2227 |
| (黄池宝, 任安祥, 李海渤, 阳年发. 高等学校化学学报, 2010, 31(11), 2222—2227) | |
| [36] | Huang C.B., Ren A. X., Acta Chim. Sinica, 2007, 65(23), 2765—2770 |
| (黄池宝, 任安祥. 化学学报, 2007, 65(23), 2765—2770) |
| [1] | XIA Jiaoyun,XU Tong,QING Jing,XIONG Yan,LIU Junjie,GONG Fuchun. Highly Sensitive Sensor for Lead Ion Based on Thioflavin T-induced G-quadruplex Formation † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1004. |
| [2] | HUANG Chibao, PAN Qi, CHEN Xiaoyuan, ZHAO Guanglian, CHEN Huashi, LIANG Xing, LÜ Guoling. Dicyanostilbene-derived Two-photon Fluorescence Probe for Mercury Ions† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1751. |
| [3] | HUANG Chibao, LIANG Xing, ZENG Qihua, CHEN Huashi, ZENG Boping, YI Daosheng, CHEN Xiaoyuan. Dicyanostilbene-derived Two-photon Fluorescence Probe for Free Zinc Ions in Live Cells and Living Tissues† [J]. Chem. J. Chinese Universities, 2015, 36(4): 646. |
| [4] | ZHANG Qian, XIA Ke, LIU Li, LIU You-Chang, ZHANG Cui, LIU Xuan, XU Huan, CHEN Shi-Jin, CHEN Ji-Da. Preparation of PVA/DTC Nanofibers and Their Adsorption Performance of Lead Ion [J]. Chem. J. Chinese Universities, 2013, 34(11): 2667. |
| [5] | XIAO Min, ZHANG Li-Na, WU Fang-Ying. Synthesis of 2'-Borono-benzaldehyde-7-(8-hydroxy-5-sulfoacid) Quinoline Hydrazone and Recognition of Pb2+ [J]. Chem. J. Chinese Universities, 2012, 33(05): 919. |
| [6] | MO Zhi-Hong*, GAO Ying-Mei, WEN Zhi-Yu, FAN Yan-Ping. Colorimetric Detection of Pb2+ Based on G-Quartet Structure by Nanoprobes [J]. Chem. J. Chinese Universities, 2010, 31(11): 2181. |
| [7] | ZOU Ming-Zhu, LU Jie, RU Qin-Hua. Studies on Zeolite Modified Electrode (Ⅱ)──Stripping Voltammograms of Ph2+ on Zeolite-Polyvinyl Electrode [J]. Chem. J. Chinese Universities, 1997, 18(10): 1618. |
| [8] | YANG Yi, GAO Ming-Yuan, MA Rong-Jiu, BIAN Feng-Lan, SHEN Jia-Cong. Studies on Molecular Diffusion Behavior Before and After Reaction Between Pb2+ -Containing Microgel and H2S in a Good Solvent [J]. Chem. J. Chinese Universities, 1994, 15(11): 1719. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||