

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (7): 1178.doi: 10.7503/cjcu20170157
• Organic Chemistry • Previous Articles Next Articles
WANG Xiaochuang, ZHANG Jie, XIE Jianwei*( )
)
Received:2017-03-16
Online:2017-07-10
Published:2017-06-20
Supported by:CLC Number:
TrendMD:
WANG Xiaochuang, ZHANG Jie, XIE Jianwei. Copper/Dodecyl Substituted Hydrazide-pyridine-N-oxide Catalyzed N-Arylation of Imidazoles with Aryliodides in Water†[J]. Chem. J. Chinese Universities, 2017, 38(7): 1178.
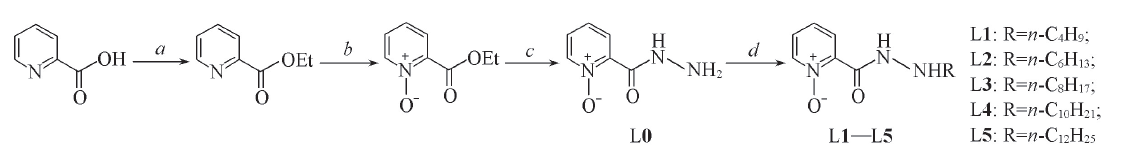
Scheme 1 Synthetic routes of target compounds L1—L5a. CH3CH2OH, SOCl2, reflux; b. m-CPBA, CH2Cl2, r.t.; c. NH2NH2·H2O, CH3CH2OH; d. (1) methylbenzene, n-Cm H 2 m + 1 CHO(m=3, 5, 7, 9, 11), reflux; (2) NaBH3CN, CH3OH(L1) or DMAB, PTS(L2—L5).

Scheme 2 Optimization of the conditions for compound 3aReaction conditions: 4-iodoanisole(0.5 mmol), imidazole(0.75 mmol), catalyst(10%, molar fraction), ligand(20%, molar fraction), base(1 mmol), solvent(1 mL), 120 ℃,12 h.
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| L1 | Dark green oil | 60 | 210.1237(210.1235) | |
| L2 | Dark green oil | 68 | 238.1550(238.1550) | |
| L3 | Dark green oil | 70 | 266.1863(266.1863) | |
| L4 | Light green solid | 70 | 49.0—51.0 | 294.2176(294.2174) |
| L5 | Light green solid | 74 | 59.0—60.0 | 322.2489(322.2489) |
Table 1 Appearances, yields and melting points of compounds L1—L5
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| L1 | Dark green oil | 60 | 210.1237(210.1235) | |
| L2 | Dark green oil | 68 | 238.1550(238.1550) | |
| L3 | Dark green oil | 70 | 266.1863(266.1863) | |
| L4 | Light green solid | 70 | 49.0—51.0 | 294.2176(294.2174) |
| L5 | Light green solid | 74 | 59.0—60.0 | 322.2489(322.2489) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| L1 | 8.40(dd, J=8.4, 2.0 Hz, 1H), 8.26(ddd, J=6.4, 1.2, 0.4 Hz, 1H), 7.48(td, J=7.6, 1.2 Hz, 1H), 7.39(ddd, J=7.6, 6.4, 2.4 Hz, 1H), 3.00(t, J=7.2 Hz, 2H), 1.62—1.55(m, 2H), 1.46—1.37(m, 2H), 0.94(t, J=7.6 Hz, 3H) | 158.1, 140.6, 140.0, 128.7, 127.4, 127.2, 52.0, 30.2, 20.3, 14.1 |
| L2 | 12.32(s, 1H), 8.34(dd,J=8.0, 2.0 Hz, 1H), 8.20(dd, J=6.4, 0.8 Hz, 1H), 7.41(td, J=7.6, 1.2 Hz, 1H), 7.35—7.31(m, 1H), 2.90(t, J=7.6 Hz, 2H), 1.55—1.48(m, 2H), 1.33—1.24(m, 6H), 0.82(t, J=6.8 Hz, 3H) | 158.1, 140.5, 134.0, 128.6, 127.3, 127.1, 52.2, 31.7, 28.1, 26.8, 22.6, 14.1 |
| L3 | 12.33(s, 1H), 8.33(dd,J=8.0, 2.0 Hz, 1H), 8.22(d, J=6.0 Hz, 1H), 7.42(t, J=7.6 Hz, 1H), 7.34(td, J=7.2, 2.0 Hz, 1H), 4.97(s, 1H), 2.90(t, J=7.2 Hz, 2H), 1.56—1.48(m, 2H), 1.33—1.20(m, 10H), 0.81(t, J=7.2 Hz, 3H) | 158.0, 140.4, 139.8, 128.5, 127.1, 127.0, 52.1, 31.7, 29.4, 29.2, 28.0, 27.0, 22.6, 14.0 |
| L4 | 12.30(s, 1H), 8.31(dd,J=8.0, 2.0 Hz, 1H), 8.20(dd, J=6.4, 0.8 Hz, 1H), 7.40(td, J=8.0, 1.2 Hz, 1H), 7.34—7.30(m, 1H), 4.98(s, 1H), 2.88(t, J=7.2 Hz, 2H), 1.53—1.46(m, 2H), 1.29—1.17(m, 14H), 0.79(t, J=7.2 Hz, 3H) | 158.0, 140.4, 139.8, 128.5, 127.1, 127.0, 52.1, 31.8, 29.6, 29.5, 29.4, 29.2, 28.0, 27.0, 22.6, 14.1 |
| L5 | 12.38(s, 1H), 8.39(dd,J=8.0, 2.0 Hz, 1H), 8.26—8.24(m, 1H), 7.46(td, J=8.0, 1.2 Hz, 1H), 7.40—7.35(m, 1H), 2.95(t, J=7.2 Hz, 2H), 1.60—1.53(m, 2H), 1.34—1.24(m, 18H), 0.86(t, J=6.8 Hz, 3H) | 158.1, 140.5, 140.0, 128.7, 127.3, 127.1, 52.3, 32.0, 29.9, 29.8, 29.7, 29.6, 29.5, 29.4, 28.2, 27.1, 22.8, 14.2 |
Table 2 1H NMR and 13C NMR data of compounds L1—L5
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| L1 | 8.40(dd, J=8.4, 2.0 Hz, 1H), 8.26(ddd, J=6.4, 1.2, 0.4 Hz, 1H), 7.48(td, J=7.6, 1.2 Hz, 1H), 7.39(ddd, J=7.6, 6.4, 2.4 Hz, 1H), 3.00(t, J=7.2 Hz, 2H), 1.62—1.55(m, 2H), 1.46—1.37(m, 2H), 0.94(t, J=7.6 Hz, 3H) | 158.1, 140.6, 140.0, 128.7, 127.4, 127.2, 52.0, 30.2, 20.3, 14.1 |
| L2 | 12.32(s, 1H), 8.34(dd,J=8.0, 2.0 Hz, 1H), 8.20(dd, J=6.4, 0.8 Hz, 1H), 7.41(td, J=7.6, 1.2 Hz, 1H), 7.35—7.31(m, 1H), 2.90(t, J=7.6 Hz, 2H), 1.55—1.48(m, 2H), 1.33—1.24(m, 6H), 0.82(t, J=6.8 Hz, 3H) | 158.1, 140.5, 134.0, 128.6, 127.3, 127.1, 52.2, 31.7, 28.1, 26.8, 22.6, 14.1 |
| L3 | 12.33(s, 1H), 8.33(dd,J=8.0, 2.0 Hz, 1H), 8.22(d, J=6.0 Hz, 1H), 7.42(t, J=7.6 Hz, 1H), 7.34(td, J=7.2, 2.0 Hz, 1H), 4.97(s, 1H), 2.90(t, J=7.2 Hz, 2H), 1.56—1.48(m, 2H), 1.33—1.20(m, 10H), 0.81(t, J=7.2 Hz, 3H) | 158.0, 140.4, 139.8, 128.5, 127.1, 127.0, 52.1, 31.7, 29.4, 29.2, 28.0, 27.0, 22.6, 14.0 |
| L4 | 12.30(s, 1H), 8.31(dd,J=8.0, 2.0 Hz, 1H), 8.20(dd, J=6.4, 0.8 Hz, 1H), 7.40(td, J=8.0, 1.2 Hz, 1H), 7.34—7.30(m, 1H), 4.98(s, 1H), 2.88(t, J=7.2 Hz, 2H), 1.53—1.46(m, 2H), 1.29—1.17(m, 14H), 0.79(t, J=7.2 Hz, 3H) | 158.0, 140.4, 139.8, 128.5, 127.1, 127.0, 52.1, 31.8, 29.6, 29.5, 29.4, 29.2, 28.0, 27.0, 22.6, 14.1 |
| L5 | 12.38(s, 1H), 8.39(dd,J=8.0, 2.0 Hz, 1H), 8.26—8.24(m, 1H), 7.46(td, J=8.0, 1.2 Hz, 1H), 7.40—7.35(m, 1H), 2.95(t, J=7.2 Hz, 2H), 1.60—1.53(m, 2H), 1.34—1.24(m, 18H), 0.86(t, J=6.8 Hz, 3H) | 158.1, 140.5, 140.0, 128.7, 127.3, 127.1, 52.3, 32.0, 29.9, 29.8, 29.7, 29.6, 29.5, 29.4, 28.2, 27.1, 22.8, 14.2 |
| Compd. | Molecular formula | GC-MS(calcd.), m/z(M+) | Compd. | Molecular formula | GC-MS(calcd.), m/z(M+) |
|---|---|---|---|---|---|
| 3a | C10H10N2O | 174(174) | 3i | C10H10N2 | 158(158) |
| 3b | C9H8N2 | 144(144) | 3j | C15H12N2 | 220(220) |
| 3c | C9H7FN2 | 162(162) | 3k | C11H10N2O | 186(186) |
| 3d | C11H12N2O | 188(188) | 3l | C15H13NO | 223(223) |
| 3e | C10H10N2O | 174(174) | 3m | C14H10ClN | 227(227) |
| 3f | C10H10N2O | 3n | C15H13NO | 223(223) | |
| 3g | C9H8N2O | 160(160) | 3o | C16H15NO | 237(237) |
| 3h | C9H7ClN2 | 178(178) | 3p | C10H8N2O2 | 188(188) |
Table 3 GC-MS data of compounds 3a—3p
| Compd. | Molecular formula | GC-MS(calcd.), m/z(M+) | Compd. | Molecular formula | GC-MS(calcd.), m/z(M+) |
|---|---|---|---|---|---|
| 3a | C10H10N2O | 174(174) | 3i | C10H10N2 | 158(158) |
| 3b | C9H8N2 | 144(144) | 3j | C15H12N2 | 220(220) |
| 3c | C9H7FN2 | 162(162) | 3k | C11H10N2O | 186(186) |
| 3d | C11H12N2O | 188(188) | 3l | C15H13NO | 223(223) |
| 3e | C10H10N2O | 174(174) | 3m | C14H10ClN | 227(227) |
| 3f | C10H10N2O | 3n | C15H13NO | 223(223) | |
| 3g | C9H8N2O | 160(160) | 3o | C16H15NO | 237(237) |
| 3h | C9H7ClN2 | 178(178) | 3p | C10H8N2O2 | 188(188) |
| Entry | Catalyst | TBAB(%, molar fraction) | Ligand | Base | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | Cu | 10 | L1 | NaOH | 42 |
| 2 | Cu | 10 | L2 | NaOH | 42 |
| 3 | Cu | 10 | L3 | NaOH | 44 |
| 4 | Cu | 10 | L4 | NaOH | 46 |
| 5 | Cu | 10 | L5 | NaOH | 50 |
| 6 | Cu | 10 | NaOH | Trace | |
| 7 | Cu | 5 | L5 | NaOH | 55 |
| 8 | Cu | 20 | L5 | NaOH | 56 |
| 9 | CuI | 5 | L5 | NaOH | 61 |
| 10 | CuBr | 5 | L5 | NaOH | 62 |
| 11 | CuCl | 5 | L5 | NaOH | 62 |
| 12 | Cu2O | 5 | L5 | NaOH | 62 |
| 13 | CuSO4 | 5 | L5 | NaOH | 60 |
| 14 | Cu(OAc)2 | 5 | L5 | NaOH | 66 |
| 15 | CuO | 5 | L5 | NaOH | 67 |
| 16 | 5 | L5 | NaOH | Trace | |
| 17 | CuO | 5 | L5 | KOH | 58 |
| 18 | CuO | 5 | L5 | K2CO3 | 48 |
| 19 | CuO | 5 | L5 | K3PO4·3H2O | 54 |
| 20 | CuO | 5 | L5 | Cs2CO3 | 44 |
| 21 | CuO | L5 | NaOH | 13 | |
| 22 | CuO | 5 | L0 | NaOH | 36 |
| 23 | CuO | 20 | L0 | NaOH | 51 |
| 24 | CuO | L0 | NaOH | Trace | |
| 25 | CuO | 5 | L5 | NaOH | 56c |
| 26 | CuO | 5 | L5 | NaOH | 88d |
Table 4 Optimization of reaction conditions for compound 3aa
| Entry | Catalyst | TBAB(%, molar fraction) | Ligand | Base | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | Cu | 10 | L1 | NaOH | 42 |
| 2 | Cu | 10 | L2 | NaOH | 42 |
| 3 | Cu | 10 | L3 | NaOH | 44 |
| 4 | Cu | 10 | L4 | NaOH | 46 |
| 5 | Cu | 10 | L5 | NaOH | 50 |
| 6 | Cu | 10 | NaOH | Trace | |
| 7 | Cu | 5 | L5 | NaOH | 55 |
| 8 | Cu | 20 | L5 | NaOH | 56 |
| 9 | CuI | 5 | L5 | NaOH | 61 |
| 10 | CuBr | 5 | L5 | NaOH | 62 |
| 11 | CuCl | 5 | L5 | NaOH | 62 |
| 12 | Cu2O | 5 | L5 | NaOH | 62 |
| 13 | CuSO4 | 5 | L5 | NaOH | 60 |
| 14 | Cu(OAc)2 | 5 | L5 | NaOH | 66 |
| 15 | CuO | 5 | L5 | NaOH | 67 |
| 16 | 5 | L5 | NaOH | Trace | |
| 17 | CuO | 5 | L5 | KOH | 58 |
| 18 | CuO | 5 | L5 | K2CO3 | 48 |
| 19 | CuO | 5 | L5 | K3PO4·3H2O | 54 |
| 20 | CuO | 5 | L5 | Cs2CO3 | 44 |
| 21 | CuO | L5 | NaOH | 13 | |
| 22 | CuO | 5 | L0 | NaOH | 36 |
| 23 | CuO | 20 | L0 | NaOH | 51 |
| 24 | CuO | L0 | NaOH | Trace | |
| 25 | CuO | 5 | L5 | NaOH | 56c |
| 26 | CuO | 5 | L5 | NaOH | 88d |
| Entry | ArX | Het-NH | Product | Compd. | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | 3a | 88 | |||
| 2 | 3b | 65 | |||
| 3 | 3c | 61c | |||
| 4 | 3d | 60 | |||
| 5 | 3e | 92 | |||
| 6 | 3f | Trace | |||
| 7 | 3g | 63 | |||
| 8 | 3h | 84 | |||
| 9 | 3i | 61d | |||
| 10 | 3j | 78 | |||
| 11 | 3k | 69 | |||
| 12 | 3l | 68 | |||
| 13 | 3m | 69 | |||
| 14 | 3n | 74 | |||
| 15 | 3o | 60 | |||
| 16 | 3p | 32 | |||
Table 5 Cu powder-catalyzed N-arylation of imidazoles with aryl halides using 2-(2-dodecylhydrazine-1-carbonyl)pyridine 1-oxide as ligand in watera
| Entry | ArX | Het-NH | Product | Compd. | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | 3a | 88 | |||
| 2 | 3b | 65 | |||
| 3 | 3c | 61c | |||
| 4 | 3d | 60 | |||
| 5 | 3e | 92 | |||
| 6 | 3f | Trace | |||
| 7 | 3g | 63 | |||
| 8 | 3h | 84 | |||
| 9 | 3i | 61d | |||
| 10 | 3j | 78 | |||
| 11 | 3k | 69 | |||
| 12 | 3l | 68 | |||
| 13 | 3m | 69 | |||
| 14 | 3n | 74 | |||
| 15 | 3o | 60 | |||
| 16 | 3p | 32 | |||
| [1] | Carmen A., Bartroli J., Belloc J., Cavalcanti F. L., Ferrando R., Gómez L. A., Ramis I., Carceller E., Merlos M., Garcia-Rafanell J., J. Med. Chem., 2004, 47(22), 5579—5582 |
| [2] | Hagberg D. P., Yum J. H., Lee H. J., Angelis F. D., Marinado T., Karlsson K. M., Humphry-Baker R., Sun L. C., Hagfeldt A., Grätzel M., Nazeeruddin M. K., J. Am. Chem. Soc., 2008, 130(19), 6259—6266 |
| [3] | Liu Y. L., Lu F., Lu P., Chem. J. Chinese Universities,2017, 38(4), 583—590 |
| (刘豫龙, 路芳, 路萍.高等学校化学学报, 2017, 38(4), 583—590) | |
| [4] | Ley S. V., Thomas A. W., Angew. Chem. Int. Ed., 2003, 42(44), 5400—5449 |
| [5] | Antilla J. C., Klapars A., Buchwald S. L., J. Am. Chem. Soc., 2002, 124(39), 11684—11688 |
| [6] | Antilla J. C., Baskin J. M., Barder T. E., Buchwald S. L., J. Org. Chem., 2004, 69(17), 5578—5587 |
| [7] | Cristau H. J., Cellier P. P., Spindler J. F., Taillefer M., Eur. J. Org. Chem., 2004, 2004(4), 695—709 |
| [8] | Monnier F., Taillefer M., Angew. Chem., 2008, 120(17), 3140—3143 |
| [9] | Beletskaya I. P., Cheprakov A. V., Coord. Chem. Rev., 2004, 248(21—24), 2337—2364 |
| [10] | Ma D. W., Cai Q., Acc. Chem. Res., 2008, 41(11), 1450—1460 |
| [11] | Monnier F., Taillefer M., Angew. Chem. Int. Ed., 2009, 48(38), 6954—6971 |
| [12] | Shen G. D., Zhao L. Y., Bao W. L., Chem. Res. Chinese Universities,2016, 32(6), 947—951 |
| [13] | Wang Y., Gao J. Y., Zhao M. D., Li J. M., Chem. Res. Chinese Universities,2015, 31(4), 549—552 |
| [14] | Carril M., SanMartin R., Dominguez E., Chem. Soc. Res., 2008, 37(4), 639—647 |
| [15] | Li Z. K., Wu Z. Q., Deng H., Zhou X. G., Chin. J. Org. Chem., 2013, 33(4), 760—770 |
| (李正凯, 吴之清, 邓杭, 周向葛.有机化学, 2013, 33(4), 760—770 | |
| [16] | Xie J. W., Zhu X. H., Huang M. N., Meng F., Chen W. W., Wan Y. Q., Eur. J. Org. Chem., 2010, 2010(17), 3219—3223 |
| [17] | Liang L., Li Z. K., Zhou X. G., Org. Lett., 2009, 11(15), 3294—3297 |
| [18] | Wu F. T., Yan N. N., Liu P., Xie J. W., Liu Y., Dai B., Tetrahedron Lett., 2014, 55(21), 3249—3251 |
| [19] | Bonnet D., Margathe J. F., Radford S., Pflimlin E., Riché S., Doman P., Hibert M., Ganesan A., ACS Comb. Sci., 2012, 14(5), 323—334 |
| [20] | Casarini M. E., Ghelfi F., Libertini E., Pagnoni U. M., Parsons A. F., Tetrahedron,2002, 58(39), 7925—7932 |
| [21] | Franc G., Jutand A., Dalton Trans., 2010, 39(34), 7873—7875 |
| [22] | Wang Y. B., Zhang Y., Yang B. B., Zhang A., Yao Q. Z., Org. Biomol. Chem., 2015, 13(15), 4101—4114 |
| [23] | Xie J. W., Zhu X. H., Huang M. N., Meng F., Chen W. W., Wan Y. Q., Eur. J. Org. Chem., 2010, 2010(17), 3219—3223 |
| [1] | JI Ding-Hao, LIU Gang, JIA Ming-Jun, ZHANG Wen-Xiang, WANG Guo-Jia, WU Tong-Hao, WANG Zhen-Lü*. Studies on Dehydrogenation of 2-Butanol over Supported Copper Catalysts Prepared by Sol-Gel and Impregnation Methods [J]. Chem. J. Chinese Universities, 2007, 28(8): 1543. |
| [2] | WANG Zhen-Lü, WU Tong-Hao, YANG Piao-Ping, YU Jian-Feng, ZHU Wan-Chun, JING Shu-Bo, LIU Guo-Zong, WANG Guo-Jia. Dehydrogenation of 2-Butanol over Supported Copper Catalysts [J]. Chem. J. Chinese Universities, 2004, 25(9): 1723. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||