

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (7): 1192.doi: 10.7503/cjcu20170042
• Organic Chemistry • Previous Articles Next Articles
ZHAO Qiqi, LIANG Miaomiao, MA Yangyang, LI Xiaokai, ZHU Huajie*( ), LI Wan*(
), LI Wan*( )
)
Received:2017-01-18
Online:2017-07-10
Published:2017-05-18
Contact:
ZHU Huajie,LI Wan
E-mail:zhuhuajie@hotmail.com;liwanjingmin@163.com
CLC Number:
TrendMD:
ZHAO Qiqi, LIANG Miaomiao, MA Yangyang, LI Xiaokai, ZHU Huajie, LI Wan. New Chiral Biscarboline N,N-Dioxide Derivatives as Catalysts over Enantioselective Hydrosilylation of Keteoimines†[J]. Chem. J. Chinese Universities, 2017, 38(7): 1192.
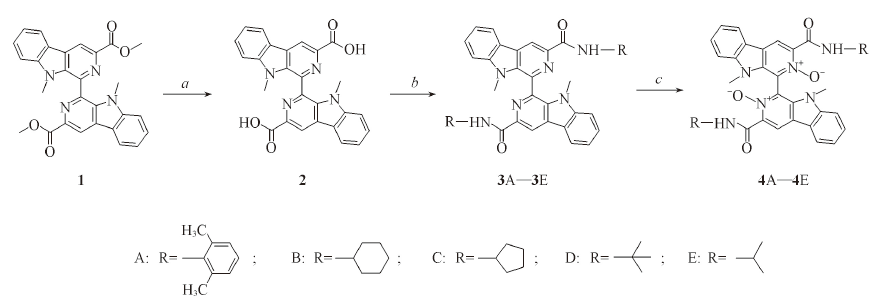
Scheme 1 Synthetic routes of chiral ligands 4A—4EReaction conditions: a. NaOH, H2O, MeOH, 60 ℃; b. (i) isobutyl-chloroformate(2.4 mmol), Et3N(2.4 mmol), DCM, 0 ℃, 20 min; (ii) amine(2.2 mmol), r. t., 12 h; c. DCM, m-CPBA(8 mmol), r. t., 24 h.
| Compd. | Appearance | Yield(%) | Elemental analysis(%, cacld. ) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 3A | White solid | 85 | 76.85(76.81) | 5.58(5.52) | 12.84(12.80) |
| 3B | White solid | 90 | 74.50(74.48) | 6.55(6.58) | 13.74(13.71) |
| 3C | White solid | 90 | 73.92(73.95) | 6.23(6.21) | 14.33(14.37) |
| 3D | White solid | 90 | 72.85(72.83) | 6.51(6.47) | 14.95(14.99) |
| 3E | White solid | 90 | 72.14(72.16) | 6.08(6.06) | 15.74(15.78) |
| 4A | White solid | 40 | 73.26(73.24) | 5.68(5.72) | 12.23(12.20) |
| 4B | White solid | 43 | 70.72(70.79) | 6.23(6.25) | 13.06(13.03) |
| 4C | White solid | 40 | 70.16(70.11) | 5.81(5.88) | 13.60(13.63) |
| 4D | White solid | 45 | 68.85(68.90) | 6.18(6.12) | 14.20(14.18) |
| 4E | White solid | 45 | 68.03(68.07) | 5.68(5.71) | 14.88(14.88) |
Table 1 Appearance, yields and elemental analysis data of compounds 3A—3E and 4A—4E
| Compd. | Appearance | Yield(%) | Elemental analysis(%, cacld. ) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 3A | White solid | 85 | 76.85(76.81) | 5.58(5.52) | 12.84(12.80) |
| 3B | White solid | 90 | 74.50(74.48) | 6.55(6.58) | 13.74(13.71) |
| 3C | White solid | 90 | 73.92(73.95) | 6.23(6.21) | 14.33(14.37) |
| 3D | White solid | 90 | 72.85(72.83) | 6.51(6.47) | 14.95(14.99) |
| 3E | White solid | 90 | 72.14(72.16) | 6.08(6.06) | 15.74(15.78) |
| 4A | White solid | 40 | 73.26(73.24) | 5.68(5.72) | 12.23(12.20) |
| 4B | White solid | 43 | 70.72(70.79) | 6.23(6.25) | 13.06(13.03) |
| 4C | White solid | 40 | 70.16(70.11) | 5.81(5.88) | 13.60(13.63) |
| 4D | White solid | 45 | 68.85(68.90) | 6.18(6.12) | 14.20(14.18) |
| 4E | White solid | 45 | 68.03(68.07) | 5.68(5.71) | 14.88(14.88) |
| Compd. | 1H NMR(CDCl3, 600 MHz), δ | 13C NMR(CDCl3, 150 MHz), δ |
|---|---|---|
| 3A | 9.44—9.36(m, 2H), 9.25(t, J=5.7 Hz, 2H), 8.33(d, J=7.9 Hz, 2H), 7.70(t, J=7.7 Hz, 2H), 7.50(d, J=8.4 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.30(t, J=7.6 Hz, 2H), 7.19(d, J=7.7 Hz, 4H), 3.54—3.47(m, 6H), 1.19(d, J=6.7 Hz, 12H) | 164.42, 146.14, 143.18, 138.73, 138.45, 137.44, 131.55, 131.44, 129.53, 128.17, 123.44, 122.25, 121.54, 121.19, 115.37, 109.83, 77.24, 77.03, 76.82, 32.39, 29.01, 23.78, 23.60 |
| 3B | 9.13(s, 2H), 8.36(s, 2H), 7.91(s, 2H), 7.68(s, 2H), 7.47(s, 2H), 7.43(t, J=7.5 Hz, 2H), 4.02(s, 2H), 3.31(s, 6H), 2.02(s, 4H), 1.69(s, 4H), 1.61(d, J=13.1 Hz, 2H), 1.44—1.37(m, 4H), 1.21(s, 4H), 1.12(d, J=30.4 Hz, 2H) | 164.11, 143.00, 139.05, 138.35, 137.13, 131.07, 129.23, 122.16, 121.50, 120.87, 114.72, 109.89, 48.38, 33.30, 32.28, 25.55, 25.08 |
| 3C | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.94(d, J=8.0 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.49—4.43(m, 2H), 3.29(s, 6H), 2.07(dt, J=13.0, 5.6 Hz, 4H), 1.68—1.63(m, 4H), 1.63—1.57(m, 4H), 1.48(dt, J=12.4, 6.2 Hz, 4H) | 164.70, 142.99, 138.99, 138.38, 137.13, 131.01, 129.26, 122.15, 121.49, 120.90, 114.69, 109.90, 51.18, 33.26, 32.22, 23.87 |
| 3D | 9.12(s, 2H), 8.32(d, J=7.9 Hz, 2H), 7.95(s, 2H), 7.68(t, J=7.6 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 3.28(d, J=8.8 Hz, 6H), 1.47(s, 18H) | 164.42, 143.00, 139.71, 138.19, 137.11, 131.07, 129.22, 122.08, 121.50, 120.85, 114.23, 109.87, 51.00, 32.18, 28.97 |
| 3E | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.85(d, J=8.3 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.47(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.35(dq, J=13.4, 6.6 Hz, 2H), 3.29(d, J=8.7 Hz, 6H), 1.24(t, J=6.1 Hz, 12H) | 164.25, 142.98, 139.00, 138.37, 137.13, 131.00, 129.26, 122.15, 121.47, 120.90, 114.71, 109.90, 41.45, 32.18, 22.81 |
| 4A | 12.68(s, 2H), 9.45(d, J=1.0 Hz, 2H), 8.25(d, J=7.9 Hz, 2H), 7.66(t, J=7.8 Hz, 2H), 7.45(dd, J=12.1, 6.4 Hz, 4H), 7.13—7.08(m, 6H), 3.40(d, J=0.6 Hz, 6H), 2.31(s, 12H) | 158.85, 143.93, 138.89, 134.79, 134.05, 132.99, 129.68, 129.32, 128.09, 127.02, 125.24, 122.96, 122.33, 121.86, 121.40, 109.97, 29.31, 18.83 |
| 4B | 11.07(d, J=7.8 Hz, 2H), 9.34(s, 2H), 8.22(d, J=7.9 Hz, 2H), 7.64—7.59(m, 2H), 7.42(t, J=7.5 Hz, 2H), 7.38(d, J=8.3 Hz, 2H), 4.08—4.00(m, 2H), 3.25(s, 6H), 2.02(dd, J=16.6, 13.2 Hz, 4H), 1.72(dd, J=13.1, 9.5 Hz, 6H), 1.60(dd, J=9.3, 3.9 Hz, 2H), 1.40(dd, J=18.5, 5.6 Hz, 4H), 1.22—1.11(m, 4H) | 159.61, 143.85, 138.52, 133.19, 129.05, 125.25, 122.63, 122.04, 121.73, 121.39, 120.89, 109.83, 48.92, 32.89, 29.48, 25.64, 24.89 |
| 4C | 11.03(t, J=14.7 Hz, 2H), 9.27(s, 2H), 8.14(d, J=7.9 Hz, 2H), 7.54(t, J=7.7 Hz, 2H), 7.34(t, J=7.5 Hz, 2H), 7.30(d, J=8.3 Hz, 2H), 4.37(dd, J=13.7, 6.9 Hz, 2H), 3.17(s, 6H), 2.00(dd, J=11.6, 4.9 Hz, 4H), 1.62(d, J=3.1 Hz, 4H), 1.51(dd, J=24.0, 13.2 Hz, 8H) | 159.12, 142.85, 137.51, 132.11, 128.06, 124.21, 121.64, 121.05, 120.70, 120.39, 119.83, 108.82, 50.58, 31.95, 28.50, 23.00 |
| 4D | 11.14(s, 2H), 9.36(d, J=1.0 Hz, 2H), 8.19(d, J=7.9 Hz, 2H), 7.63(t, J=7.7 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.39(d, J=8.2 Hz, 2H), 3.25(d, J=0.5 Hz, 6H), 1.51(s, 18H) | 159.38, 143.85, 138.51, 133.80, 129.01, 125.22, 122.63, 122.02, 121.60, 121.40, 120.51, 109.81, 51.49, 29.51, 28.77 |
| 4E | 11.00(d, J=7.5 Hz, 2H), 9.35(s, 2H), 8.21(d, J=7.9 Hz, 2H), 7.61(dd, J=11.4, 4.1 Hz, 2H), 7.42(t, J=7.5 Hz, 2H), 7.37(d, J=8.3 Hz, 2H), 4.33(dd, J=13.8, 6.7 Hz, 2H), 3.24(s, 6H), 1.27(dd, J=6.6, 3.2 Hz, 12H) | 159.69, 143.87, 138.51, 133.17, 129.10, 125.23, 122.68, 122.09, 121.71, 121.39, 120.88, 109.83, 41.99, 29.54, 22.61 |
Table 2 1H NMR and 13C NMR data of compounds 3A—3E and 4A—4E
| Compd. | 1H NMR(CDCl3, 600 MHz), δ | 13C NMR(CDCl3, 150 MHz), δ |
|---|---|---|
| 3A | 9.44—9.36(m, 2H), 9.25(t, J=5.7 Hz, 2H), 8.33(d, J=7.9 Hz, 2H), 7.70(t, J=7.7 Hz, 2H), 7.50(d, J=8.4 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.30(t, J=7.6 Hz, 2H), 7.19(d, J=7.7 Hz, 4H), 3.54—3.47(m, 6H), 1.19(d, J=6.7 Hz, 12H) | 164.42, 146.14, 143.18, 138.73, 138.45, 137.44, 131.55, 131.44, 129.53, 128.17, 123.44, 122.25, 121.54, 121.19, 115.37, 109.83, 77.24, 77.03, 76.82, 32.39, 29.01, 23.78, 23.60 |
| 3B | 9.13(s, 2H), 8.36(s, 2H), 7.91(s, 2H), 7.68(s, 2H), 7.47(s, 2H), 7.43(t, J=7.5 Hz, 2H), 4.02(s, 2H), 3.31(s, 6H), 2.02(s, 4H), 1.69(s, 4H), 1.61(d, J=13.1 Hz, 2H), 1.44—1.37(m, 4H), 1.21(s, 4H), 1.12(d, J=30.4 Hz, 2H) | 164.11, 143.00, 139.05, 138.35, 137.13, 131.07, 129.23, 122.16, 121.50, 120.87, 114.72, 109.89, 48.38, 33.30, 32.28, 25.55, 25.08 |
| 3C | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.94(d, J=8.0 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.49—4.43(m, 2H), 3.29(s, 6H), 2.07(dt, J=13.0, 5.6 Hz, 4H), 1.68—1.63(m, 4H), 1.63—1.57(m, 4H), 1.48(dt, J=12.4, 6.2 Hz, 4H) | 164.70, 142.99, 138.99, 138.38, 137.13, 131.01, 129.26, 122.15, 121.49, 120.90, 114.69, 109.90, 51.18, 33.26, 32.22, 23.87 |
| 3D | 9.12(s, 2H), 8.32(d, J=7.9 Hz, 2H), 7.95(s, 2H), 7.68(t, J=7.6 Hz, 2H), 7.46(d, J=8.3 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 3.28(d, J=8.8 Hz, 6H), 1.47(s, 18H) | 164.42, 143.00, 139.71, 138.19, 137.11, 131.07, 129.22, 122.08, 121.50, 120.85, 114.23, 109.87, 51.00, 32.18, 28.97 |
| 3E | 9.14(s, 2H), 8.35(d, J=7.9 Hz, 2H), 7.85(d, J=8.3 Hz, 2H), 7.68(t, J=7.7 Hz, 2H), 7.47(d, J=8.3 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 4.35(dq, J=13.4, 6.6 Hz, 2H), 3.29(d, J=8.7 Hz, 6H), 1.24(t, J=6.1 Hz, 12H) | 164.25, 142.98, 139.00, 138.37, 137.13, 131.00, 129.26, 122.15, 121.47, 120.90, 114.71, 109.90, 41.45, 32.18, 22.81 |
| 4A | 12.68(s, 2H), 9.45(d, J=1.0 Hz, 2H), 8.25(d, J=7.9 Hz, 2H), 7.66(t, J=7.8 Hz, 2H), 7.45(dd, J=12.1, 6.4 Hz, 4H), 7.13—7.08(m, 6H), 3.40(d, J=0.6 Hz, 6H), 2.31(s, 12H) | 158.85, 143.93, 138.89, 134.79, 134.05, 132.99, 129.68, 129.32, 128.09, 127.02, 125.24, 122.96, 122.33, 121.86, 121.40, 109.97, 29.31, 18.83 |
| 4B | 11.07(d, J=7.8 Hz, 2H), 9.34(s, 2H), 8.22(d, J=7.9 Hz, 2H), 7.64—7.59(m, 2H), 7.42(t, J=7.5 Hz, 2H), 7.38(d, J=8.3 Hz, 2H), 4.08—4.00(m, 2H), 3.25(s, 6H), 2.02(dd, J=16.6, 13.2 Hz, 4H), 1.72(dd, J=13.1, 9.5 Hz, 6H), 1.60(dd, J=9.3, 3.9 Hz, 2H), 1.40(dd, J=18.5, 5.6 Hz, 4H), 1.22—1.11(m, 4H) | 159.61, 143.85, 138.52, 133.19, 129.05, 125.25, 122.63, 122.04, 121.73, 121.39, 120.89, 109.83, 48.92, 32.89, 29.48, 25.64, 24.89 |
| 4C | 11.03(t, J=14.7 Hz, 2H), 9.27(s, 2H), 8.14(d, J=7.9 Hz, 2H), 7.54(t, J=7.7 Hz, 2H), 7.34(t, J=7.5 Hz, 2H), 7.30(d, J=8.3 Hz, 2H), 4.37(dd, J=13.7, 6.9 Hz, 2H), 3.17(s, 6H), 2.00(dd, J=11.6, 4.9 Hz, 4H), 1.62(d, J=3.1 Hz, 4H), 1.51(dd, J=24.0, 13.2 Hz, 8H) | 159.12, 142.85, 137.51, 132.11, 128.06, 124.21, 121.64, 121.05, 120.70, 120.39, 119.83, 108.82, 50.58, 31.95, 28.50, 23.00 |
| 4D | 11.14(s, 2H), 9.36(d, J=1.0 Hz, 2H), 8.19(d, J=7.9 Hz, 2H), 7.63(t, J=7.7 Hz, 2H), 7.43(t, J=7.5 Hz, 2H), 7.39(d, J=8.2 Hz, 2H), 3.25(d, J=0.5 Hz, 6H), 1.51(s, 18H) | 159.38, 143.85, 138.51, 133.80, 129.01, 125.22, 122.63, 122.02, 121.60, 121.40, 120.51, 109.81, 51.49, 29.51, 28.77 |
| 4E | 11.00(d, J=7.5 Hz, 2H), 9.35(s, 2H), 8.21(d, J=7.9 Hz, 2H), 7.61(dd, J=11.4, 4.1 Hz, 2H), 7.42(t, J=7.5 Hz, 2H), 7.37(d, J=8.3 Hz, 2H), 4.33(dd, J=13.8, 6.7 Hz, 2H), 3.24(s, 6H), 1.27(dd, J=6.6, 3.2 Hz, 12H) | 159.69, 143.87, 138.51, 133.17, 129.10, 125.23, 122.68, 122.09, 121.71, 121.39, 120.88, 109.83, 41.99, 29.54, 22.61 |
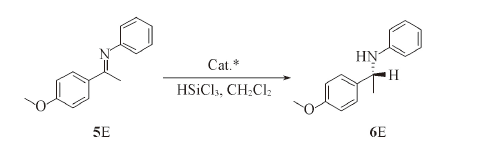
Scheme 2 Enantioselective hydrosilylation of ketoimine 5E catalyzed by compounds 4A—4EAll the reactions were performed using compound 5E(0.1 mmol) and HSiCl3(0.2 mmol) in the presence of compound 4 at -20 ℃ for 16 h.
| Cat. | Yield(%) | e. e.(%) | Cat. | Yield(%) | e. e.(%) |
|---|---|---|---|---|---|
| 4A | 80 | 40 | 4D | 95 | 19 |
| 4B | 98 | 50 | 4E | 90 | 21 |
| 4C | 90 | 35 |
Table 3 Enantioselective hydrosilylation of ketoimine 5E catalyzed by compounds 4A—4E*
| Cat. | Yield(%) | e. e.(%) | Cat. | Yield(%) | e. e.(%) |
|---|---|---|---|---|---|
| 4A | 80 | 40 | 4D | 95 | 19 |
| 4B | 98 | 50 | 4E | 90 | 21 |
| 4C | 90 | 35 |
| Compd. | R1 | R2 | Yield(%) | e. e.(%) |
|---|---|---|---|---|
| 5A | H | H | 95 | 57 |
| 5B | H | MeO | 90 | 68 |
| 5C | H | Br | 85 | 45 |
| 5B | NO2 | H | 93 | 40 |
| 5E | MeO | H | 98 | 63 |
Table 4 Enantioselective hydrosilylation of ketoimines 5A—5E catalyzed by catalyst 4B
| Compd. | R1 | R2 | Yield(%) | e. e.(%) |
|---|---|---|---|---|
| 5A | H | H | 95 | 57 |
| 5B | H | MeO | 90 | 68 |
| 5C | H | Br | 85 | 45 |
| 5B | NO2 | H | 93 | 40 |
| 5E | MeO | H | 98 | 63 |
| [1] | Jones S., Warner C., Org. Biomol. Chem., 2012, 10, 2189—2200 |
| [2] | Wang Z. Y., Ye X. X., Wei S. Y., Wu P. C., Zhang A. J., Sun J., Org. Lett., 2006, 8, 999—1001 |
| [3] | Wang Z. Y., Wang C., Zhou L., Sun J., Org. Lett., 2013, 11, 787—797 |
| [4] | Feringa B. L., Van Delden R. A., Angew. Chem. Int. Ed., 1999, 38, 3418—3428 |
| [5] | Jang H., Liu W., Zhang S. X., Liao W. W., Chem. Res. Chinese Universities,2016, 32(2), 385—389 |
| [6] | Blaser H. U., Malan C., Pugin B., Spindler F., Studer M., Adv. Synth. Catal., 2003, 345, 103—151 |
| [7] | Pan W., Deng Y., He J. B., Bai B., Zhu H. J., Tetrahedron,2013, 69, 7253—7257 |
| [8] | Kobayashi S., Ishitani H., Chem. Rev., 1999, 99, 1069—1094 |
| [9] | Yu Z., Jin W., Jiang Q., Angew. Chem. Int. Ed., 2012, 51, 6060—6072 |
| [10] | Xie J. H., Zhu S. F., Zhou Q. L., Chem. Soc.Rev. ,2012, 41, 4126—4139 |
| [11] | Cobley C. J., Henschke J. P., Adv. Synth. Catal., 2003, 345, 195—201 |
| [12] | Noyor R., Hashiguchi S., Acc. Chem. Res., 1997, 30, 97—102 |
| [13] | Moessner C., Bolm C., Angew. Chem. Int. Ed., 2006, 117, 7736—7739 |
| [14] | Kobayashi S., Yasuda M., Hachiya L., Chem. Lett., 1996, 5, 407—408 |
| [15] | Iwasaki F., Onomura O., Mishima K., Kanematsu T., Maki T., Matsumura Y., Tetrahedron Lett., 2001, 42, 2525—2527 |
| [16] | Baudequin C., Chaturvedi D., Tsoggoeva S. B., Eur. J. Org. Chem., 2007, 16, 2623—2629 |
| [17] | Zhang Z., Rooshenas P., Hausmann H., Schreiner P. R., Synthesis,2009, 9, 1531—1539 |
| [18] | Kanemitsu T., Umehara A., HaneJi R., Nagata K., Itoh T., Tetrahedron,2012, 20, 3893—3898 |
| [19] | Bai B., Shen L., Ren J., Zhu H. J., Adv. Synth. Catal., 2012, 354, 354—358 |
| [20] | Shen Y. C., Li Y. X., Wen X. J., Feng X. M., Chin. J. Org. Chem., 2005, 25, 272—283 |
| (申永存, 李燕霞, 闻雪敬, 冯小明. 有机化学, 2005, 25, 272—283) | |
| [21] | Liu X. H., Lin L. L., Feng X. M., Org. Chem. Front., 2014, 3, 298—302 |
| [22] | Deng Y., Pan W., Pei Y. N., Li J. L., Bai B., Zhu H. J., Tetrahedron,2013, 68, 10431—10437 |
| [23] | Tang W., Zhang X., Chem. Rev., 2003, 103, 3029—3069 |
| [24] | Feng J., Lin L., Yu K., Liu X., Feng X. M., Adv. Synth. Catal., 2015, 357, 1305—1310 |
| [25] | Feng J., Fu X., Chen Z., Lin L., Liu X., Feng X. M., Org. Lett. ,2013, 15, 2640—2643 |
| [26] | Liu X. H., Lin L. L., Feng X. M., Acc. Chem. Res., 2011, 44, 574—587 |
| [27] | Liu L., Yang Q., Yu H., Li J. L., Pei Y. N., Zhu H. J., Tetrahedron,2015, 71, 3296—3302 |
| [28] | Pei Y. N., Deng Y., Li J. L., Liu L., Zhu H. J., Tetrahedron Lett., 2014, 55, 2948—2952 |
| [29] | Pan W., Ma W. G., Yang X. D., Zheng Y. H., Song B. Q., Niu Y. Z., Gu J., Hu D. B., Yang Q., Zhu H. J., Chem. J. Chinese Universites,2015, 36(2), 325—329 |
| (潘威, 马文广, 杨晓东, 郑昀晔, 宋碧清, 牛永志, 古吉, 胡栋宝, 杨芹, 朱华结.高等学校化学学报, 2015, 36(2), 325—329) |
| [1] | ZHU Jielian, XIA Xiaofeng, LIANG Minting, LIU Xiang, LI Hexing. Asymmetric Hydrosilation of Aromatic Ketones Catalyzed by CuFe2O4 Nanoparticles† [J]. Chem. J. Chinese Universities, 2016, 37(3): 539. |
| [2] | XU Guo-Hua, Higashitani Ko . Formation of OTS Self-assembled Monolayer on Glass Surface Investigated by AFM [J]. Chem. J. Chinese Universities, 2000, 21(8): 1257. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||